Hexagen Skin and Nasal Antiseptic
Hexagen by
Drug Labeling and Warnings
Hexagen by is a Otc medication manufactured, distributed, or labeled by Global Health Solutions DBA Turn Therapeutics. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
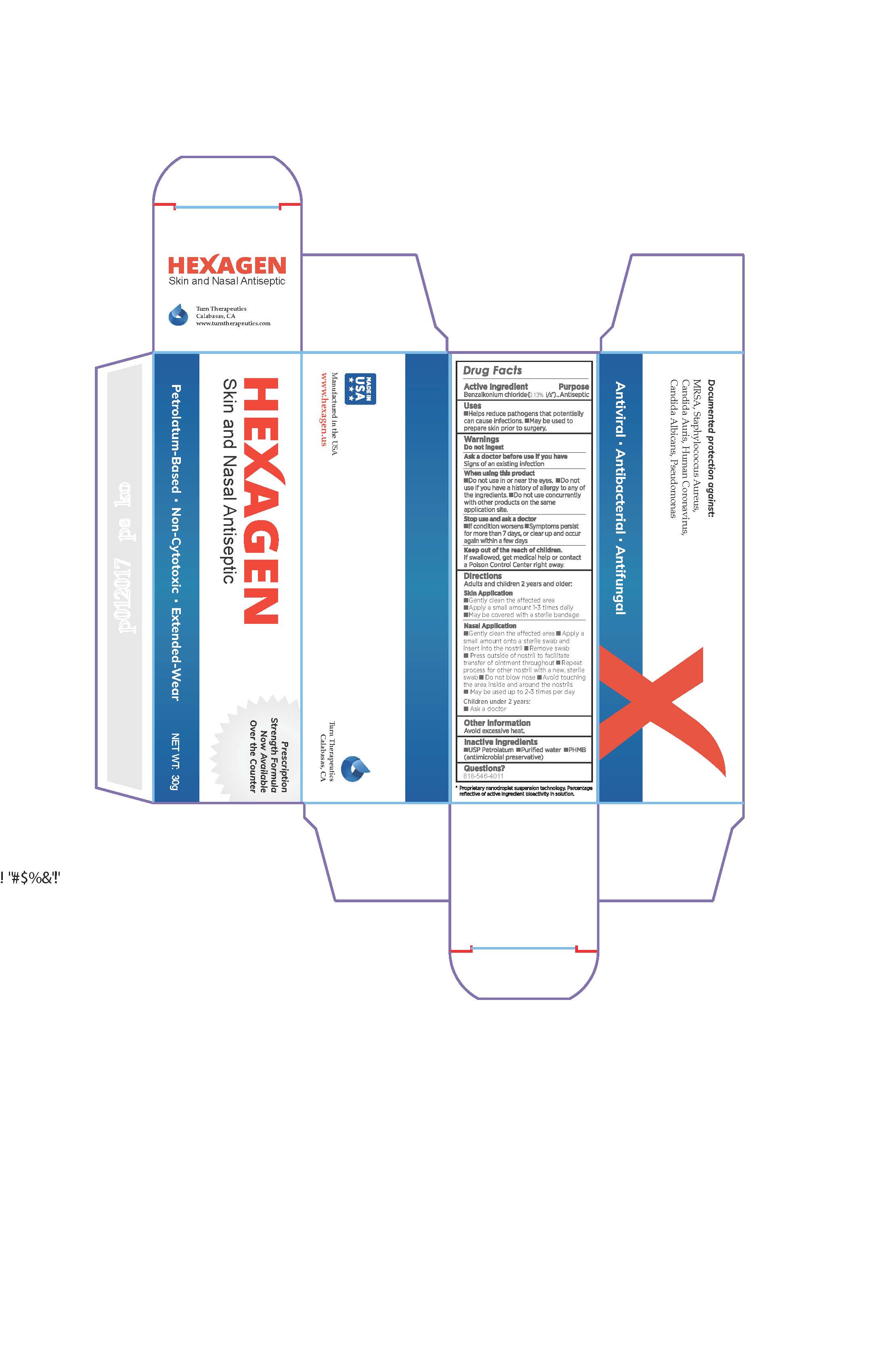
HEXAGEN- skin and nasal antiseptic ointment
Global Health Solutions DBA Turn Therapeutics
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Hexagen Skin and Nasal Antiseptic
Uses
- Help reduce pathogens that potentiallu can cause infections.
- May be used to prepare skin prior to surgery.
When using this product
- Do not use in or near the eyes.
- Do not use if you have a history of allergy to any of the ingredients.
- Do no use concurrently with other products on the same application site.
Stop use and ask a doctor
- If condition worsens
- Symptoms persist for more than 7 days, or clear up and occur again within a few days.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Adults and children 2 years and older:
Skin Application
- Gently clean the affected area
- Apply a small amount 1-3 times daily
- May be covered with a sterile bandage
Nasal Application
- Gently clean the affected area
- Apply a small amount onto a sterile swab and insert into the nostril
- Remove swab
- Press outside of nostril to facilitate transfer of ointment throughout
- Repeat process for other nostril with a new, sterile swab
- Do not blow nose
- Avoid touching the area inside and around the nostrils
- May be used up to 2-3 times per day
Children under 2 years:
- Ask a doctor
*Proprietary nanodroplet suspension technology. Percentage reflective of active ingredient bioactivity in solution.
HEXAGEN
Skin and Nasal Antiseptic
Prescription Strength Formula Now Available Over the Counter
Petrolatum-Based, Non-Cytotoxic, Extended-Wear NET WT: 30 g
Made in USA
Manufactured in the USA
www.hexagen.us
Turn Therapeutics
Calabasas, CA
Documented protection against:
MRSA, Staphylococcus Aureus, Candida Auris, Human Coronavirus, Candida Albicans, Pseudomonas
Antiviral, Antibacterial, Antifungal
| HEXAGEN
skin and nasal antiseptic ointment |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Global Health Solutions DBA Turn Therapeutics (079695890) |
| Registrant - Global Health Solutions DBA Turn Therapeutics (079695890) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Global Health Solutions DBA Turn Therapeutics | 079695890 | manufacture(69578-268) , label(69578-268) | |
Trademark Results [Hexagen]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 HEXAGEN 97880804 not registered Live/Pending |
Affera, Inc 2023-04-10 |
 HEXAGEN 88238897 not registered Live/Pending |
MORPHOGEN NUTRITION LLC 2018-12-21 |
 HEXAGEN 87334512 5285038 Live/Registered |
Bradley E. Burnam 2017-02-14 |
 HEXAGEN 79386704 not registered Live/Pending |
Libertine FPE Limited 2023-07-25 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.