Esomeprazole Magnesium Delayed-Release Capsules USP, 20 mg
esomeprazole magnesium by
Drug Labeling and Warnings
esomeprazole magnesium by is a Otc medication manufactured, distributed, or labeled by Graviti Pharmaceuticals Private Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ESOMEPRAZOLE MAGNESIUM - esomeprazole magnesium capsule, delayed release
Graviti Pharmaceuticals Private Limited
----------
Esomeprazole Magnesium Delayed-Release Capsules USP, 20 mg
Esomeprazole Magnesium Drug Facts
Active ingredient (in each capsule)
Esomeprazole 20 mg
(Each delayed-release capsule corresponds to 22.250 mg esomeprazole magnesium trihydrate)
Purpose
Acid reducer
Uses
- treats frequent heartburn (occurs 2 or more days a week)
- not intended for immediate relief of heartburn; this drug may take 1 to 4 days for full effect
Allergy alert:
- Do not use if you are allergic to esomeprazole.
- Esomeprazole may cause severe skin reactions. Symptoms may include:
- Skin reddening
- Blisters
- Rash
If an allergic reaction occurs, stop use and seek medical help right away.
Do not use if you have
- trouble or pain swallowing food, vomiting with blood, or bloody or black stools
- heartburn with lightheadedness , sweating or dizziness
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- frequent chest pain
These may be signs of a serious condition. See your doctor.
Ask a doctor before use if you have
- had heartburn over 3 months. This may be a sign of a more serious condition.
- frequent wheezing, particularly with heartburn
- unexplained weight loss
- nausea or vomiting
- stomach pain
Ask a doctor or pharmacist before use if you are taking a prescription drug. Acid reducers may interact with certain prescription drugs.
Stop use and ask a doctor if
- your heartburn continues or worsens
- you need to take this product for more than 14 days
- you need to take more than 1 course of treatment every 4 months
- you get diarrhea
- you develop a rash or joint pain
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- adults 18 years of age and older
- this product is to be used once a day (every 24 hours), every day for 14 days
- may take 1 to 4 days for full effect
14-Day Course of Treatment Repeated 14-Day Courses (if needed)
- swallow 1 capsule with a glass of water before eating in the morning
- take every day for 14 days
- do not take more than 1 capsule a day
- swallow whole. Do not crush or chew capsules.
- do not use for more than 14 days unless directed by your doctor
- you may repeat a 14-day course every 4 months
- do not take for more than 14 days or more often than every 4 months unless directed by a doctor
- children under 18 years of age: ask a doctor before use. Heartburn in children may sometimes be caused by a serious condition.
- read the directions and warnings before use
- keep the carton. It contains important information.
- Store at 20° to 25°C (68° to 77°F).
Esomeprazole Magnesium Delayed-Release Capsules USP, 20 mg:
FD&C Blue 2, Gelatin, Hydroxypropyl Cellulose, Hypromellose, Magnesium Stearate, Methacrylic Acid and Ethyl Acrylate Copolymer Dispersion, Mono-and Di-Glycerides, Pharmaceutical Ink, Polysorbate 80, Sugar Spheres, Talc, Triethyl Citrate.
Questions or comments?
Call Toll-free weekdays 9AM to 5PM EST at 1-855-298-4506
Contact Graviti Pharmaceuticals Inc., or www.gravitipharma.com; or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
Made in India
Additional Information
KEEP CARTON FOR COMPLETE WARNINGS AND IMPORTANT INFORMATION.
Do Not Use if seal under bottle cap imprinted with "SEALED for YOUR PORTECTION" or Blue band around the center of each capsule is broken or missing.
Tips for Managing Heartburn
- Avoid foods or drinks that are more likely to cause heartburn, such as rich, spicy, fatty and fried foods, chocolate, caffeine, alcohol and even some acidic fruits and vegetables.
- Eat slowly and do not eat big meals.
- Do not eat late at night or just before bedtime.
- Do not lie flat or bend over soon after eating.
- Raise the head of your bed.
- Wear loose-fitting clothing around your stomach.
- If you are overweight, lose weight.
- If you smoke, quit smoking.
Graviti Pharmaceuticals Inc.,
Wilmington, Delaware-19801,
USA.
Made in India
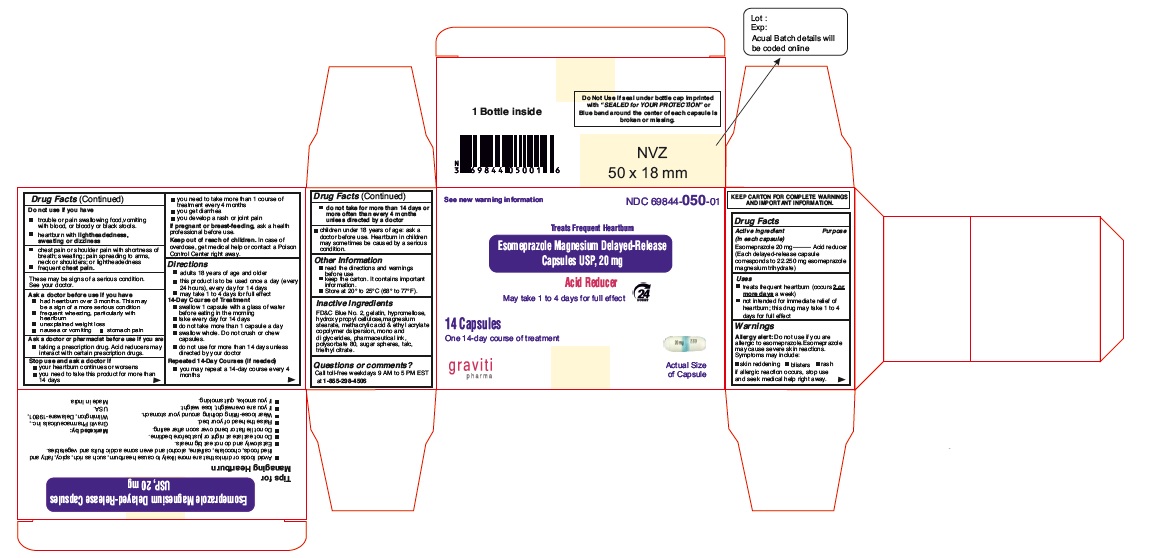
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Principal Display Panel - 20 mg Capsules: Container Label
NDC: 69844-050-01
Esomeprazole Magnesium Delayed-Release Capsules USP, 20 mg
Acid Reducer
Treats Frequent Heartburn
May take 1 to 4 Days for Full effect
24 HR
14 Capsules
One 14-day Course of Treatment
See New Warning Information
Manufactured for Graviti Pharmaceuticals Inc.
Made in India.

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Principal Display Panel - 14 Capsule Carton label
NDC: 69844-050-01
Esomeprazole Magnesium Delayed-Release Capsules USP, 20 mg
Acid Reducer
Treats Frequent Heartburn
May take 1 to 4 Days for Full effect
24 HR
14 Capsules
One 14-day Course of Treatment
See New Warning Information
Manufactured for Graviti Pharmaceuticals Inc.
Made in India.
| ESOMEPRAZOLE MAGNESIUM
esomeprazole magnesium capsule, delayed release |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Graviti Pharmaceuticals Private Limited (650884781) |
| Registrant - Graviti Pharmaceuticals Private Limited (650884781) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Graviti Pharmaceuticals Private Limited | 650884781 | MANUFACTURE(69844-050) , ANALYSIS(69844-050) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.