COPAXONE- glatiramer acetate injection, solution
Copaxone by
Drug Labeling and Warnings
Copaxone by is a Prescription medication manufactured, distributed, or labeled by Teva Neuroscience, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use COPAXONE® safely and effectively. See full prescribing information for COPAXONE.
COPAXONE (glatiramer acetate injection) for subcutaneous use
Initial U.S. Approval: 1996RECENT MAJOR CHANGES
Indications and Usage (1) 7/2019
INDICATIONS AND USAGE
COPAXONE is indicated for the treatment of relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults (1).
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
Known hypersensitivity to glatiramer acetate or mannitol (4)
WARNINGS AND PRECAUTIONS
- Immediate Post-Injection Reaction (flushing, chest pain, palpitations, tachycardia, anxiety, dyspnea, throat constriction, and/or urticaria), may occur within seconds to minutes after injection and are generally transient and self-limiting (5.1)
- Chest pain, usually transient (5.2)
- Lipoatrophy and skin necrosis may occur. Instruct patients in proper injection technique and to rotate injection sites (5.3)
- COPAXONE can modify immune response (5.4)
ADVERSE REACTIONS
- In controlled studies of COPAXONE 20 mg/mL, most common adverse reactions (≥10% and ≥1.5 times higher than placebo) were: injection site reactions, vasodilatation, rash, dyspnea, and chest pain (6.1)
- In a controlled study of COPAXONE 40 mg/mL, most common adverse reactions (≥10% and ≥1.5 times higher than placebo) were: injection site reactions (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Teva Pharmaceuticals at 1-888-483-8279 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 1/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
RECENT MAJOR CHANGES
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose
2.2 Instructions for Use
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Immediate Post-Injection Reaction
5.2 Chest Pain
5.3 Lipoatrophy and Skin Necrosis
5.4 Potential Effects on Immune Response
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Use in Patients with Impaired Renal Function
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
Copaxone® (glatiramer acetate injection) 20 mg/mL, 30 Single-Dose Pre-Filled Syringe Carton Text
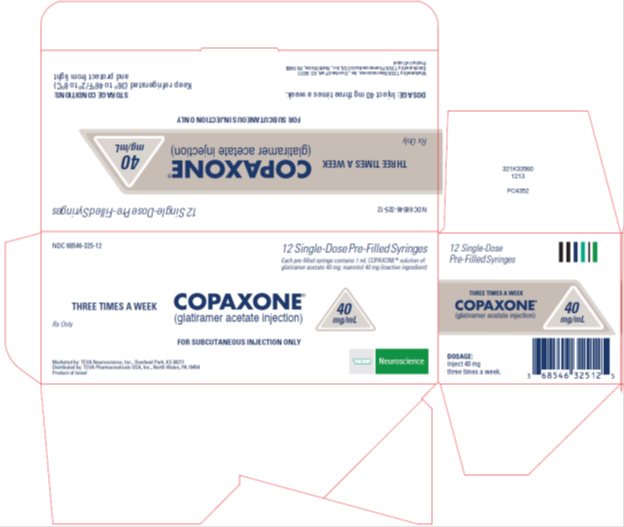
Copaxone® (glatiramer acetate injection) 40 mg/mL, 12 Single-Dose Pre-Filled Syringe Carton Text
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose
COPAXONE is for subcutaneous use only. Do not administer intravenously. The dosing schedule depends on the product strength that is selected. The recommended doses are:
- COPAXONE 20 mg per mL: administer once per day
or
- COPAXONE 40 mg per mL: administer three times per week and at least 48 hours apart
COPAXONE 20 mg per mL and COPAXONE 40 mg per mL are not interchangeable.
2.2 Instructions for Use
Remove one blister-packaged prefilled syringe from the refrigerated carton. Let the prefilled syringe stand at room temperature for 20 minutes to allow the solution to warm to room temperature. Visually inspect the syringe for particulate matter and discoloration prior to administration. The solution in the syringe should appear clear, colorless to slightly yellow. If particulate matter or discoloration is observed, discard the syringe.
Areas for subcutaneous self-injection include arms, abdomen, hips, and thighs. The prefilled syringe is for single use only. Discard unused portions.
- COPAXONE 20 mg per mL: administer once per day
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Immediate Post-Injection Reaction
Approximately 16% of patients exposed to COPAXONE 20 mg per mL in the 5 placebo-controlled trials compared to 4% of those on placebo, and approximately 2% of patients exposed to COPAXONE 40 mg per mL in a placebo-controlled trial compared to none on placebo, experienced a constellation of symptoms that may occur immediately (within seconds to minutes, with the majority of symptoms observed within 1 hour) after injection and included at least two of the following: flushing, chest pain, palpitations, tachycardia, anxiety, dyspnea, constriction of the throat, and urticaria. In general, these symptoms have their onset several months after the initiation of treatment, although they may occur earlier, and a given patient may experience one or several episodes of these symptoms. Whether or not any of these symptoms actually represent a specific syndrome is uncertain. Typically, the symptoms were transient and self-limited and did not require treatment; however, there have been reports of patients with similar symptoms who received emergency medical care. Whether an immunologic or nonimmunologic mechanism mediates these episodes, or whether several similar episodes seen in a given patient have identical mechanisms, is unknown.
5.2 Chest Pain
Approximately 13% of COPAXONE 20 mg per mL patients in the 5 placebo-controlled studies compared to 6% of placebo patients, and approximately 2% of patients exposed to COPAXONE 40 mg per mL in a placebo-controlled trial compared to 1% of placebo patients, experienced at least one episode of transient chest pain. While some of these episodes occurred in the context of the Immediate Post-Injection Reaction described above, many did not. The temporal relationship of this chest pain to an injection was not always known. The pain was usually transient, often unassociated with other symptoms, and appeared to have no clinical sequelae. Some patients experienced more than one such episode, and episodes usually began at least 1 month after the initiation of treatment. The pathogenesis of this symptom is unknown.
5.3 Lipoatrophy and Skin Necrosis
At injection sites, localized lipoatrophy and, rarely, injection site skin necrosis may occur. Lipoatrophy occurred in approximately 2% of patients exposed to COPAXONE 20 mg per mL in the 5 placebo-controlled trials compared to none on placebo, and 0.5% of patients exposed to COPAXONE 40 mg per mL in a single placebo-controlled trial and none on placebo. Skin necrosis has only been observed in the postmarketing setting. Lipoatrophy may occur at various times after treatment onset (sometimes after several months) and is thought to be permanent. There is no known therapy for lipoatrophy. To assist in possibly minimizing these events, the patient should be advised to follow proper injection technique and to rotate injection sites with each injection.
5.4 Potential Effects on Immune Response
Because COPAXONE can modify immune response, it may interfere with immune functions. For example, treatment with COPAXONE may interfere with the recognition of foreign antigens in a way that would undermine the body's tumor surveillance and its defenses against infection. There is no evidence that COPAXONE does this, but there has not been a systematic evaluation of this risk. Because COPAXONE is an antigenic material, it is possible that its use may lead to the induction of host responses that are untoward, but systematic surveillance for these effects has not been undertaken.
Although COPAXONE is intended to minimize the autoimmune response to myelin, there is the possibility that continued alteration of cellular immunity due to chronic treatment with COPAXONE may result in untoward effects.
Glatiramer acetate-reactive antibodies are formed in most patients receiving glatiramer acetate. Studies in both the rat and monkey have suggested that immune complexes are deposited in the renal glomeruli. Furthermore, in a controlled trial of 125 RRMS patients given COPAXONE 20 mg per mL, subcutaneously every day for 2 years, serum IgG levels reached at least 3 times baseline values in 80% of patients by 3 months of initiation of treatment. By 12 months of treatment, however, 30% of patients still had IgG levels at least 3 times baseline values, and 90% had levels above baseline by 12 months. The antibodies are exclusively of the IgG subtype and predominantly of the IgG-1 subtype. No IgE type antibodies could be detected in any of the 94 sera tested; nevertheless, anaphylaxis can be associated with the administration of most any foreign substance, and therefore, this risk cannot be excluded.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Immediate Post-Injection Reaction [see Warnings and Precautions (5.1)]
- Chest Pain [see Warnings and Precautions (5.2)]
- Lipoatrophy and Skin Necrosis [see Warnings and Precautions (5.3)]
- Potential Effects on Immune Response [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Incidence in Controlled Clinical Trials
COPAXONE 20 mg per mL per day
Among 563 patients treated with COPAXONE in blinded placebo-controlled trials, approximately 5% of the subjects discontinued treatment because of an adverse reaction. The adverse reactions most commonly associated with discontinuation were: injection site reactions, dyspnea, urticaria, vasodilatation, and hypersensitivity. The most common adverse reactions were: injection site reactions, vasodilatation, rash, dyspnea, and chest pain.
Table 1 lists signs and symptoms that occurred in at least 2% of patients treated with COPAXONE 20 mg per mL in the placebo-controlled trials. These signs and symptoms were numerically more common in patients treated with COPAXONE than in patients treated with placebo. Adverse reactions were usually mild in intensity.
Table 1: Adverse Reactions in Controlled Clinical Trials with an Incidence ≥2% of Patients and More Frequent with COPAXONE (20 mg per mL Daily) than with Placebo COPAXONE
20 mg/mL (n=563)
%
Placebo
(n=564)
%
Blood And Lymphatic System Disorders
Lymphadenopathy
7
3
Cardiac Disorders
Palpitations
9
4
Tachycardia
5
2
Eye Disorders
Eye Disorder
3
1
Diplopia
3
2
Gastrointestinal Disorders
Nausea
15
11
Vomiting
7
4
Dysphagia
2
1
General Disorders And Administration Site Conditions
Injection Site Erythema
43
10
Injection Site Pain
40
20
Injection Site Pruritus
27
4
Injection Site Mass
26
6
Asthenia
22
21
Pain
20
17
Injection Site Edema
19
4
Chest Pain
13
6
Injection Site Inflammation
9
1
Edema
8
2
Injection Site Reaction
8
1
Pyrexia
6
5
Injection Site Hypersensitivity
4
0
Local Reaction
3
1
Chills
3
1
Face Edema
3
1
Edema Peripheral
3
2
Injection Site Fibrosis
2
1
Injection Site Atrophy*
2
0
Immune System Disorders
Hypersensitivity
3
2
Infections And Infestations
Infection
30
28
Influenza
14
13
Rhinitis
7
5
Bronchitis
6
5
Gastroenteritis
6
4
Vaginal Candidiasis
4
2
Metabolism And Nutrition Disorders
Weight Increased
3
1
Musculoskeletal And Connective Tissue Disorders
Back Pain
12
10
Neoplasms Benign, Malignant And Unspecified (Incl Cysts And Polyps)
Benign Neoplasm of Skin
2
1
Nervous System Disorders
Tremor
4
2
Migraine
4
2
Syncope
3
2
Speech Disorder
2
1
Psychiatric Disorders
Anxiety
13
10
Nervousness
2
1
Renal And Urinary Disorders
Micturition Urgency
5
4
Respiratory, Thoracic And Mediastinal Disorders
Dyspnea
14
4
Cough
6
5
Laryngospasm
2
1
Skin And Subcutaneous Tissue Disorders
Rash
19
11
Hyperhidrosis
7
5
Pruritus
5
4
Urticaria
3
1
Skin Disorder
3
1
Vascular Disorders
Vasodilatation
20
5
*Injection site atrophy comprises terms relating to localized lipoatrophy at injection site
Adverse reactions which occurred only in 4 to 5 more subjects in the COPAXONE group than in the placebo group (less than 1% difference), but for which a relationship to COPAXONE could not be excluded, were arthralgia and herpes simplex.
Laboratory analyses were performed on all patients participating in the clinical program for COPAXONE. Clinically-significant laboratory values for hematology, chemistry, and urinalysis were similar for both COPAXONE and placebo groups in blinded clinical trials. In controlled trials one patient discontinued treatment due to thrombocytopenia (16 x109/L), which resolved after discontinuation of treatment.
Data on adverse reactions occurring in the controlled clinical trials of COPAXONE 20 mg per mL were analyzed to evaluate differences based on sex. No clinically-significant differences were identified. Ninety-six percent of patients in these clinical trials were Caucasian. The majority of patients treated with COPAXONE were between the ages of 18 and 45. Consequently, data are inadequate to perform an analysis of the adverse reaction incidence related to clinically-relevant age subgroups.
Other Adverse Reactions
In the paragraphs that follow, the frequencies of less commonly reported adverse clinical reactions are presented. Because the reports include reactions observed in open and uncontrolled premarketing studies (n= 979), the role of COPAXONE in their causation cannot be reliably determined. Furthermore, variability associated with adverse reaction reporting, the terminology used to describe adverse reactions, etc., limit the value of the quantitative frequency estimates provided. Reaction frequencies are calculated as the number of patients who used COPAXONE and reported a reaction divided by the total number of patients exposed to COPAXONE. All reported reactions are included except those already listed in the previous table, those too general to be informative, and those not reasonably associated with the use of the drug. Reactions are further classified within body system categories and enumerated in order of decreasing frequency using the following definitions: Frequent adverse reactions are defined as those occurring in at least 1/100 patients and infrequent adverse reactions are those occurring in 1/100 to 1/1,000 patients.
Body as a Whole:
Frequent: Abscess
Infrequent: Injection site hematoma, moon face, cellulitis, hernia, injection site abscess, serum sickness, suicide attempt, injection site hypertrophy, injection site melanosis, lipoma, and photosensitivity reaction.
Cardiovascular:
Frequent: Hypertension.
Infrequent: Hypotension, midsystolic click, systolic murmur, atrial fibrillation, bradycardia, fourth heart sound, postural hypotension, and varicose veins.
Digestive:
Infrequent: Dry mouth, stomatitis, burning sensation on tongue, cholecystitis, colitis, esophageal ulcer, esophagitis, gastrointestinal carcinoma, gum hemorrhage, hepatomegaly, increased appetite, melena, mouth ulceration, pancreas disorder, pancreatitis, rectal hemorrhage, tenesmus, tongue discoloration, and duodenal ulcer.
Endocrine:
Infrequent: Goiter, hyperthyroidism, and hypothyroidism.
Gastrointestinal:
Frequent: Bowel urgency, oral moniliasis, salivary gland enlargement, tooth caries, and ulcerative stomatitis.
Hemic and Lymphatic:
Infrequent: Leukopenia, anemia, cyanosis, eosinophilia, hematemesis, lymphedema, pancytopenia, and splenomegaly.
Metabolic and Nutritional:
Infrequent: Weight loss, alcohol intolerance, Cushing’s syndrome, gout, abnormal healing, and xanthoma.
Musculoskeletal:
Infrequent: Arthritis, muscle atrophy, bone pain, bursitis, kidney pain, muscle disorder, myopathy, osteomyelitis, tendon pain, and tenosynovitis.
Nervous:
Frequent: Abnormal dreams, emotional lability, and stupor.
Infrequent: Aphasia, ataxia, convulsion, circumoral paresthesia, depersonalization, hallucinations, hostility, hypokinesia, coma, concentration disorder, facial paralysis, decreased libido, manic reaction, memory impairment, myoclonus, neuralgia, paranoid reaction, paraplegia, psychotic depression, and transient stupor.
Respiratory:
Frequent: Hyperventilation and hay fever.
Infrequent: Asthma, pneumonia, epistaxis, hypoventilation, and voice alteration.
Skin and Appendages:
Frequent: Eczema, herpes zoster, pustular rash, skin atrophy, and warts.
Infrequent: Dry skin, skin hypertrophy, dermatitis, furunculosis, psoriasis, angioedema, contact dermatitis, erythema nodosum, fungal dermatitis, maculopapular rash, pigmentation, benign skin neoplasm, skin carcinoma, skin striae, and vesiculobullous rash.
Special Senses:
Frequent: Visual field defect.
Infrequent: Dry eyes, otitis externa, ptosis, cataract, corneal ulcer, mydriasis, optic neuritis, photophobia, and taste loss.
Urogenital:
Frequent: Amenorrhea, hematuria, impotence, menorrhagia, suspicious papanicolaou smear, urinary frequency, and vaginal hemorrhage.
Infrequent: Vaginitis, flank pain (kidney), abortion, breast engorgement, breast enlargement, carcinoma in situ cervix, fibrocystic breast, kidney calculus, nocturia, ovarian cyst, priapism, pyelonephritis, abnormal sexual function, and urethritis.
COPAXONE 40 mg per mL three times per week
Among 943 patients treated with COPAXONE 40 mg per mL three times per week in a blinded, placebo-controlled trial, approximately 3% of the subjects discontinued treatment because of an adverse reaction. The most common adverse reactions were injection site reactions, which were also the most common cause of discontinuation.
Table 2 lists signs and symptoms that occurred in at least 2% of patients treated with COPAXONE 40 mg per mL in the blinded, placebo-controlled trial. These signs and symptoms were numerically more common in patients treated with COPAXONE 40 mg per mL than in patients treated with placebo. Adverse reactions were usually mild in intensity.
Table 2: Adverse Reactions in a Controlled Clinical Trial with an Incidence ≥2% of Patients and More Frequent with COPAXONE (40 mg per mL Three Times per Week) than with Placebo COPAXONE
40 mg/mL
(n=943)
%
Placebo
(n=461)
%
General Disorders And Administration Site Conditions
Injection Site Erythema
22
2
Injection Site Pain
10
2
Injection Site Mass
6
0
Injection Site Pruritus
6
0
Injection Site Edema
6
0
Pyrexia
3
2
Influenza-like Illness
3
2
Injection Site Inflammation
2
0
Chills
2
0
Chest Pain
2
1
Infections And Infestations
Nasopharyngitis
11
9
Respiratory Tract Infection Viral
3
2
Respiratory, Thoracic and Mediastinal Disorders
Dyspnea
3
0
Vascular Disorders
Vasodilatation
3
0
Gastrointestinal Disorders
Nausea
2
1
Skin And Subcutaneous Tissue Disorders
Erythema
2
0
Rash
2
1
No new adverse reactions appeared in subjects treated with COPAXONE 40 mg per mL three times per week as compared to subjects treated with COPAXONE 20 mg per mL per day in clinical trials and during postmarketing experience. Data on adverse reactions occurring in the controlled clinical trial of COPAXONE 40 mg per mL were analyzed to evaluate differences based on sex. No clinically significant differences were identified. Ninety-eight percent of patients in this clinical trial were Caucasian and the majority were between the ages of 18 and 50. Consequently, data are inadequate to perform an analysis of the adverse reaction incidence related to clinically-relevant age groups.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of COPAXONE. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Body as a Whole: sepsis; SLE syndrome; hydrocephalus; enlarged abdomen; allergic reaction; anaphylactoid reaction
Cardiovascular System: thrombosis; peripheral vascular disease; pericardial effusion; myocardial infarct; deep thrombophlebitis; coronary occlusion; congestive heart failure; cardiomyopathy; cardiomegaly; arrhythmia; angina pectoris
Digestive System: tongue edema; stomach ulcer; hemorrhage; liver function abnormality; liver damage; hepatitis; eructation; cirrhosis of the liver; cholelithiasis
Hemic and Lymphatic System: thrombocytopenia; lymphoma-like reaction; acute leukemia
Metabolic and Nutritional Disorders: hypercholesterolemia
Musculoskeletal System: rheumatoid arthritis; generalized spasm
Nervous System: myelitis; meningitis; CNS neoplasm; cerebrovascular accident; brain edema; abnormal dreams; aphasia; convulsion; neuralgia
Respiratory System: pulmonary embolus; pleural effusion; carcinoma of lung
Special Senses: glaucoma; blindness
Urogenital System: urogenital neoplasm; urine abnormality; ovarian carcinoma; nephrosis; kidney failure; breast carcinoma; bladder carcinoma; urinary frequency
-
7 DRUG INTERACTIONS
Interactions between COPAXONE and other drugs have not been fully evaluated. Results from existing clinical trials do not suggest any significant interactions of COPAXONE with therapies commonly used in MS patients, including the concurrent use of corticosteroids for up to 28 days. COPAXONE has not been formally evaluated in combination with interferon beta.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available human data on the use of COPAXONE in pregnant women are not sufficient to support conclusions about drug-associated risk for major birth defects and miscarriage. Administration of glatiramer acetate by subcutaneous injection to pregnant rats and rabbits resulted in no adverse effects on embryofetal or offspring development (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Human Data
There are no adequate and well-controlled studies of COPAXONE in pregnant women. The available postmarketing reports, case series, and small cohort studies do not provide sufficient information to support conclusions about drug-associated risk for major birth defects and miscarriage.
Animal Data
In rats or rabbits receiving glatiramer acetate by subcutaneous injection during the period of organogenesis, no adverse effects on embryofetal development were observed at doses up to 37.5 mg/kg/day (18 and 36 times, respectively, the therapeutic human dose of 20 mg/day on a mg/m2 basis). In rats receiving subcutaneous glatiramer acetate at doses of up to 36 mg/kg from day 15 of pregnancy throughout lactation, no significant effects on delivery or on offspring growth and development were observed.
8.2 Lactation
Risk Summary
There are no data on the presence of glatiramer acetate in human milk, the effects on breastfed infants, or the effects on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for COPAXONE and any potential adverse effects on the breastfed infant from COPAXONE or from the underlying maternal condition.
-
11 DESCRIPTION
Glatiramer acetate, the active ingredient of COPAXONE, consists of the acetate salts of synthetic polypeptides, containing four naturally occurring amino acids: L-glutamic acid, L-alanine, L-tyrosine, and L-lysine with an average molar fraction of 0.141, 0.427, 0.095, and 0.338, respectively. The average molecular weight of glatiramer acetate is 5,000 – 9,000 daltons. Glatiramer acetate is identified by specific antibodies.
Chemically, glatiramer acetate is designated L-glutamic acid polymer with L-alanine, L-lysine and L-tyrosine, acetate (salt). Its structural formula is:
(Glu, Ala, Lys, Tyr)x●xCH3COOH
(C5H9NO4●C3H7NO2●C6H14N2O2●C9H11NO3)x●xC2H4O2
CAS - 147245-92-9
COPAXONE is a clear, colorless to slightly yellow, sterile, nonpyrogenic solution for subcutaneous injection. Each 1 mL of COPAXONE solution contains 20 mg or 40 mg of glatiramer acetate and the following inactive ingredient: 40 mg of mannitol. The pH of the solutions is approximately 5.5 to 7.0. The biological activity of glatiramer acetate is determined by its ability to block the induction of experimental autoimmune encephalomyelitis (EAE) in mice.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism(s) by which glatiramer acetate exerts its effects in patients with MS are not fully understood. However, glatiramer acetate is thought to act by modifying immune processes that are believed to be responsible for the pathogenesis of MS. This hypothesis is supported by findings of studies that have been carried out to explore the pathogenesis of experimental autoimmune encephalomyelitis, a condition induced in animals through immunization against central nervous system derived material containing myelin and often used as an experimental animal model of MS. Studies in animals and in vitro systems suggest that upon its administration, glatiramer acetate-specific suppressor T-cells are induced and activated in the periphery.
Because glatiramer acetate can modify immune functions, concerns exist about its potential to alter naturally-occurring immune responses. There is no evidence that glatiramer acetate does this, but this has not been systematically evaluated [see Warnings and Precautions (5.4)].
12.3 Pharmacokinetics
Results obtained in pharmacokinetic studies performed in humans (healthy volunteers) and animals support that a substantial fraction of the therapeutic dose delivered to patients subcutaneously is hydrolyzed locally. Larger fragments of glatiramer acetate can be recognized by glatiramer acetate-reactive antibodies. Some fraction of the injected material, either intact or partially hydrolyzed, is presumed to enter the lymphatic circulation, enabling it to reach regional lymph nodes, and some may enter the systemic circulation intact.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In a 2-year carcinogenicity study, mice were administered up to 60 mg/kg/day glatiramer acetate by subcutaneous injection (up to 15 times the human therapeutic dose of 20 mg/day on a mg/m2 basis). No increase in systemic neoplasms was observed. In males receiving the 60-mg/kg/day dose, there was an increased incidence of fibrosarcomas at the injection sites. These sarcomas were associated with skin damage precipitated by repetitive injections of an irritant over a limited skin area.
In a 2-year carcinogenicity study, rats were administered up to 30 mg/kg/day glatiramer acetate by subcutaneous injection (up to 15 times the human therapeutic dose on a mg/m2 basis). No increase in neoplasms was observed.
Mutagenesis
Glatiramer acetate was not mutagenic in in vitro (Ames test, mouse lymphoma tk) assays. Glatiramer acetate was clastogenic in two separate in vitro chromosomal aberration assays in cultured human lymphocytes but not clastogenic in an in vivo mouse bone marrow micronucleus assay.
Impairment of Fertility
When glatiramer acetate was administered by subcutaneous injection prior to and during mating (males and females) and throughout gestation and lactation (females) at doses up to 36 mg/kg/day (18 times the human therapeutic dose on a mg/m2 basis) no adverse effects were observed on reproductive or developmental parameters.
-
14 CLINICAL STUDIES
Evidence supporting the effectiveness of COPAXONE derives from five placebo-controlled trials, four of which used a COPAXONE dose of 20 mg per mL per day and one of which used a COPAXONE dose of 40 mg per mL three times per week.
COPAXONE 20 mg per mL per day
Study 1 was performed at a single center. Fifty patients were enrolled and randomized to receive daily doses of either COPAXONE, 20 mg per mL subcutaneously, or placebo (COPAXONE: n=25; placebo: n=25). Patients were diagnosed with RRMS by standard criteria, and had at least 2 exacerbations during the 2 years immediately preceding enrollment. Patients were ambulatory, as evidenced by a score of no more than 6 on the Kurtzke Disability Scale Score (DSS), a standard scale ranging from 0–Normal to 10–Death due to MS. A score of 6 is defined as one at which a patient is still ambulatory with assistance; a score of 7 means the patient must use a wheelchair.
Patients were examined every 3 months for 2 years, as well as within several days of a presumed exacerbation. To confirm an exacerbation, a blinded neurologist had to document objective neurologic signs, as well as document the existence of other criteria (e.g., the persistence of the neurological signs for at least 48 hours).
The protocol-specified primary outcome measure was the proportion of patients in each treatment group who remained exacerbation free for the 2 years of the trial, but two other important outcomes were also specified as endpoints: the frequency of attacks during the trial, and the change in the number of attacks compared with the number which occurred during the previous 2 years.
Table 3 presents the values of the three outcomes described above, as well as several protocol-specified secondary measures. These values are based on the intent-to-treat population (i.e., all patients who received at least 1 dose of treatment and who had at least 1 on-treatment assessment):
Table 3: Study 1 Efficacy Results COPAXONE
20 mg/mL
(n=25)
Placebo
(n=25)
P-Value
% Relapse-Free Patients
14/25 (56%)
7/25 (28%)
0.085
Mean Relapse Frequency
0.6/2 years
2.4/2 years
0.005
Reduction in Relapse Rate Compared to Prestudy
3.2
1.6
0.025
Median Time to First Relapse (days)
>700
150
0.03
% of Progression-Free* Patients
20/25 (80%)
13/25 (52%)
0.07
*Progression was defined as an increase of at least 1 point on the DSS, persisting for at least 3 consecutive months.
Study 2 was a multicenter trial of similar design which was performed in 11 US centers. A total of 251 patients (COPAXONE: n=125; placebo: n=126) were enrolled. The primary outcome measure was the Mean 2-Year Relapse Rate. Table 4 presents the values of this outcome for the intent-to-treat population, as well as several secondary measures:
Table 4: Study 2 Efficacy Results COPAXONE
20 mg/mL
(n=125)
Placebo
(n=126)
P-Value
Mean No. of Relapses
1.19/2 years
1.68 /2 years
0.055
% Relapse-Free Patients
42/125 (34%)
34/126 (27%)
0.25
Median Time to First Relapse (days)
287
198
0.23
% of Progression-Free Patients
98/125 (78%)
95/126 (75%)
0.48
Mean Change in DSS
-0.05
+0.21
0.023
In both studies, COPAXONE exhibited a clear beneficial effect on relapse rate, and it is based on this evidence that COPAXONE is considered effective.
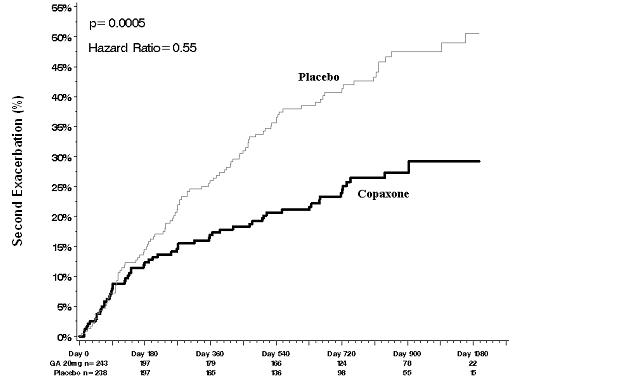
In Study 3, 481 patients who had recently (within 90 days) experienced an isolated demyelinating event and who had lesions typical of multiple sclerosis on brain MRI were randomized to receive either COPAXONE 20 mg per mL (n=243) or placebo (n=238). The primary outcome measure was time to development of a second exacerbation. Patients were followed for up to three years or until they reached the primary endpoint. Secondary outcomes were brain MRI measures, including number of new T2 lesions and T2 lesion volume.
Time to development of a second exacerbation was significantly delayed in patients treated with COPAXONE compared to placebo (Hazard Ratio = 0.55; 95% confidence interval 0.40 to 0.77; Figure 1). The Kaplan-Meier estimates of the percentage of patients developing a relapse within 36 months were 42.9% in the placebo group and 24.7% in the COPAXONE group.
Figure 1: Time to Second Exacerbation
Patients treated with COPAXONE demonstrated fewer new T2 lesions at the last observation (rate ratio 0.41; confidence interval 0.28 to 0.59; p < 0.0001). Additionally, baseline-adjusted T2 lesion volume at the last observation was lower for patients treated with COPAXONE (ratio of 0.89; confidence interval 0.84 to 0.94; p = 0.0001).
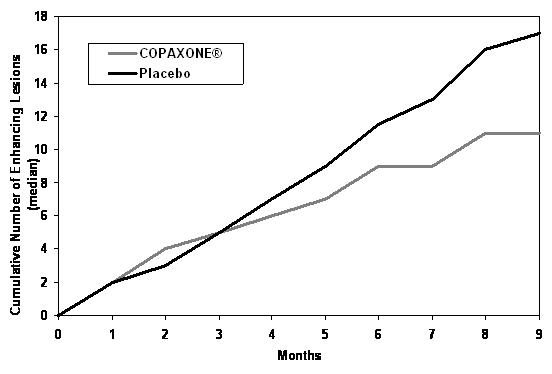
Study 4 was a multinational study in which MRI parameters were used both as primary and secondary endpoints. A total of 239 patients with RRMS (COPAXONE: n=119; and placebo: n=120) were randomized. Inclusion criteria were similar to those in the second study with the additional criterion that patients had to have at least one Gd-enhancing lesion on the screening MRI. The patients were treated in a double-blind manner for nine months, during which they underwent monthly MRI scanning. The primary endpoint for the double-blind phase was the total cumulative number of T1 Gd-enhancing lesions over the nine months. Table 5 summarizes the results for the primary outcome measure monitored during the trial for the intent-to-treat cohort.
Table 5: Study 4 MRI Results COPAXONE
20 mg/mL (n=119)
Placebo
(n=120)
P-Value
Medians of the Cumulative Number of T1 Gd-Enhancing Lesions
11
17
0.0030
Figure 2 displays the results of the primary outcome on a monthly basis.
Figure 2: Median Cumulative Number of Gd-Enhancing Lesions
COPAXONE 40 mg per mL three times per week
Study 5 was a double-blind, placebo-controlled, multinational study with a total of 1404 patients with RRMS randomized in a 2:1 ratio to receive either COPAXONE 40 mg per mL (n=943) or placebo (n=461) three times a week for 12 months. Patients had a median of 2 relapses in the 2 years prior to screening and had not received any interferon-beta for at least 2 months prior to screening. Baseline EDSS scores ranged from 0 to 5.5 with a median of 2.5. Neurological evaluations were performed at baseline, every three months, and at unscheduled visits for suspected relapse or early termination. MRI was performed at baseline, months 6 and 12, or early termination. A total of 91% of those assigned to COPAXONE and 93% of those assigned to placebo completed treatment at 12 months.
The primary outcome measure was the total number of confirmed relapses (persistence of neurological symptoms for at least 48 hours confirmed on examination with objective signs). The effect of COPAXONE on several magnetic resonance imaging (MRI) variables, including number of new or enlarging T2 lesions and number of enhancing lesions on T1-weighted images, was also measured at months 6 and 12.
Table 6 presents the results for the intent-to-treat population.
Table 6: Study 5 Efficacy and MRI Results COPAXONE
40 mg/mL
(n=943)
Placebo
(n=461)
P-Value
Clinical Endpoints
Number of confirmed relapses during the 12-month placebo-controlled phase
Adjusted Mean Estimates
Relative risk reduction
0.331
34%
0.505
<0.0001
MRI Endpoints
Cumulative number of new or enlarging T2 lesions at Months 6 and12
Adjusted Mean Estimates
Relative risk reduction
3.650
35%
5.592
<0.0001
Cumulative number of enhancing lesions on T1-weighted images at Months 6 and 12
Adjusted Mean Estimates
Relative risk reduction
0.905
45%
1.639
<0.0001
-
16 HOW SUPPLIED/STORAGE AND HANDLING
COPAXONE (glatiramer acetate injection) is a clear, colorless to slightly yellow, sterile, nonpyrogenic solution supplied as:
- 20 mg per mL in a single-dose, prefilled syringe with a white plunger, in individual blister packages supplied in 30-count cartons (NDC: 68546-317-30).
- 40 mg per mL in a single-dose, prefilled syringe with a blue plunger, in individual blister packages supplied in 12-count cartons (NDC: 68546-325-12).
Store COPAXONE refrigerated at 2°C to 8°C (36°F to 46°F). If needed, the patient may store COPAXONE at room temperature, 15°C to 30°C (59°F to 86°F), for up to one month, but refrigeration is preferred. Avoid exposure to higher temperatures or intense light. Do not freeze COPAXONE. If a COPAXONE syringe freezes, it should be discarded.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Immediate Post-Injection Reaction
Advise patients that COPAXONE may cause various symptoms after injection, including flushing, chest pain, palpitations, tachycardia, anxiety, dyspnea, constriction of the throat, and urticaria. These symptoms occur within seconds to minutes after injection and are generally transient and self-limited and do not require specific treatment. Inform patients that these symptoms may occur early or may have their onset several months after the initiation of treatment. A patient may experience one or several episodes of these symptoms.
Chest Pain
Advise patients that they may experience transient chest pain either as part of the Immediate Post-Injection Reaction or in isolation. Inform patients that the pain should be transient. Some patients may experience more than one such episode, usually beginning at least one month after the initiation of treatment. Patients should be advised to seek medical attention if they experience chest pain of unusual duration or intensity.
Lipoatrophy and Skin Necrosis at Injection Site
Advise patients that localized lipoatrophy, and rarely, skin necrosis may occur at injection sites. Instruct patients to follow proper injection technique and to rotate injection areas and sites with each injection to minimize these risks.
Pregnancy
Instruct patients that if they are pregnant or plan to become pregnant while taking COPAXONE they should inform their physician [see Use in Specific Populations (8.1)].
Lactation
Advise patients to notify their healthcare provider if they are breastfeeding or intend to breastfeed during COPAXONE therapy [see Use in Specific Populations (8.2)].
Instructions for Use
Instruct patients to read the COPAXONE Patient Information leaflet carefully. COPAXONE 20 mg per mL and COPAXONE 40 mg per mL are not interchangeable. COPAXONE 20 mg per mL is administered daily and COPAXONE 40 mg per mL is administered three times per week. Caution patients to use aseptic technique. The first injection should be performed under the supervision of a health care professional. Instruct patients to rotate injection areas and sites with each injection. Caution patients against the reuse of needles or syringes. Instruct patients in safe disposal procedures.
Storage Conditions
Advise patients that the recommended storage condition for COPAXONE is refrigeration at 36oF to 46oF (2oC to 8oC). If needed, the patient may store COPAXONE at room temperature, 59oF to 86oF (15oC to 30oC), for up to one month, but refrigeration is preferred. COPAXONE should not be exposed to higher temperatures or intense light. Do not freeze COPAXONE.

Marketed by: Teva Neuroscience, Inc., Parsippany, NJ 07054
Distributed by: Teva Pharmaceuticals USA, Inc., Parsippany, NJ 07054©2019 Teva Neuroscience, Inc.
COP-006
-
Patient Information
COPAXONE (co-PAX-own)
(glatiramer acetate injection)
for subcutaneous use
Read this Patient Information before you start using COPAXONE and each time you get a refill. There may be new information. This information does not take the place of talking with your doctor about your medical condition or your treatment.
What is COPAXONE?
COPAXONE is a prescription medicine that is used to treat relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.
It is not known if COPAXONE is safe and effective in children under 18 years of age.
Who should not use COPAXONE?
- Do not use COPAXONE if you are allergic to glatiramer acetate, mannitol or any of the ingredients in COPAXONE. See the end of this leaflet for a complete list of the ingredients in COPAXONE.
What should I tell my doctor before using COPAXONE?
Before you use COPAXONE, tell your doctor if you:
- are pregnant or plan to become pregnant. It is not known if COPAXONE will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if COPAXONE passes into your breast milk. Talk to your doctor about the best way to feed your baby while using COPAXONE.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
COPAXONE may affect the way other medicines work, and other medicines may affect how COPAXONE works.
Know the medicines you take. Keep a list of your medicines with you to show your doctor and pharmacist when you get a new medicine.
How should I use COPAXONE?
- For detailed instructions, see the Instructions for Use at the end of this leaflet for complete information on how to use COPAXONE.
- Your doctor will tell you how much COPAXONE to use and when to use it.
- COPAXONE is given by injection under your skin (subcutaneously).
- Use COPAXONE exactly as your doctor tells you to use it.
- Since every body type is different, talk with your doctor about the injection areas that are best for you.
- You should receive your first dose of COPAXONE with a doctor or nurse present. This might be at your doctor’s office or with a visiting home health nurse who will teach you how to give your COPAXONE injections.
What are the possible side effects of COPAXONE?
COPAXONE may cause serious side effects, including:
-
Immediate Post-Injection Reactions. Serious side effects may happen right after or within minutes after you inject COPAXONE at any time during your course of treatment. Call your doctor right away if you have any of these immediate post-injection reaction symptoms including:
- redness to your cheeks or other parts of the body (flushing)
- chest pain
- fast heart beat
- anxiety
- breathing problems or tightness in your throat
- swelling, rash, hives, or itching
If you have symptoms of an immediate post-injection reaction, do not give yourself more injections until a doctor tells you to.
- Chest Pain. You can have chest pain as part of an immediate post-injection reaction or by itself. This type of chest pain usually lasts a few minutes and can begin around 1 month after you start using COPAXONE. Call your doctor right away if you have chest pain while using COPAXONE.
-
Damage to your skin. Damage to the fatty tissue just under your skin’s surface (lipoatrophy) and, rarely, death of your skin tissue (necrosis) can happen when you use COPAXONE. Damage to the fatty tissue under your skin can cause a “dent” at the injection site that may not go away. You can reduce your chance of developing these problems by:
- following your doctor’s instructions for how to use COPAXONE
- choosing a different injection area each time you use COPAXONE. See Step 4 in the Instructions for Use, “Choose your injection area”.
The most common side effects of COPAXONE include:
- skin problems at your injection site including:
- redness
- pain
- swelling
- itching
- lumps
- rash
- shortness of breath
- flushing (vasodilation)
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of COPAXONE. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store COPAXONE?
- Store COPAXONE in the refrigerator between 36°F to 46°F (2°C to 8°C).
- When you are not able to refrigerate COPAXONE, you may store it for up to 1 month at room temperature between 59°F to 86°F (15°C to 30°C).
- Protect COPAXONE from light or high temperature.
- Do not freeze COPAXONE syringes. If a syringe freezes, throw it away in a sharps disposal container. See Step 13 in the Instructions for Use, “Dispose of your needles and syringes”.
Keep COPAXONE and all medicines out of the reach of children.
General information about the safe and effective use of COPAXONE.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information Leaflet. Do not use COPAXONE for a condition for which it was not prescribed. Do not give COPAXONE to other people, even if they have the same symptoms as you have. It may harm them.
This Patient Information Leaflet summarizes the most important information about COPAXONE. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about COPAXONE that is written for health professionals.
For more information, go to www.copaxone.com or call 1-800-887-8100.
What are the ingredients in COPAXONE?
Active ingredient: glatiramer acetate
Inactive ingredients: mannitol
COPPL-004
Revised: July 2019 -
Instructions for Use
COPAXONE (co-PAX-own)
(glatiramer acetate injection)
for subcutaneous use
For subcutaneous injection only.
Do not inject COPAXONE in your veins (intravenously).
Do not re-use your COPAXONE prefilled syringes.
Do not share your COPAXONE prefilled syringes with another person. You may give another person an infection or get an infection from them.
You should receive your first dose of COPAXONE with a doctor or nurse present. This might be at your doctor’s office or with a visiting home health nurse who will show you how to give your own injections.
COPAXONE comes in either a 20 mg Prefilled Syringe with needle attached or a 40 mg Prefilled Syringe with needle attached. How often a dose is given depends on the product strength that is prescribed. Your doctor will prescribe the correct dose for you.
Instructions for Using Your COPAXONE 20 mg Prefilled Syringe:
- COPAXONE 20 mg is injected 1 time each day, in the fatty layer under your skin (subcutaneously).
- Each COPAXONE 20 mg prefilled syringe is for single use (1 time use) only.
- The COPAXONE 20 mg dose is packaged in boxes of 30 prefilled syringes with needles attached. COPAXONE 20 mg prefilled syringes have white plungers.
Instructions for Using Your COPAXONE 40 mg Prefilled Syringe:
- COPAXONE 40 mg is injected 3 times each week, in the fatty layer under your skin (subcutaneously).
- COPAXONE 40 mg should be given on the same 3 days each week, if possible for example, Monday, Wednesday, and Friday. Give your COPAXONE injections at least 48 hours (2 days) apart.
- Each COPAXONE 40 mg prefilled syringe is for single use (1 time use) only.
- The COPAXONE 40 mg dose is packaged in boxes of 12 prefilled syringes with needles attached. COPAXONE 40 mg prefilled syringes have blue plungers.
How do I inject COPAXONE?
Step 1: Gather the supplies you will need to inject COPAXONE. See Figure A.
- 1 blister pack with a COPAXONE Prefilled Syringe with needle attached
- Alcohol wipe (not supplied)
- Dry cotton ball (not supplied)
- A place to record your injections, like a notebook (not supplied)
- Sharps disposal container (not supplied). See Step 13 below, “Dispose of your needles and syringes”.

Figure A
Step 2: Remove only 1 blister pack from the COPAXONE prefilled syringe carton. See Figure B.
Figure B
- Place the supplies you will need on a clean, flat surface in a well-lit area.
- After you remove 1 blister pack from the carton, keep all unused syringes in the carton and store them in the refrigerator.
- Let the blister pack, with the syringe inside, warm to room temperature for about 20 minutes.
- Wash your hands. Be careful not to touch your face or hair after washing your hands.
Step 3: Look closely at your COPAXONE prefilled syringe.
- There may be small air bubbles in the syringe. Do not try to push the air bubble from the syringe before giving your injection so you do not lose any medicine.
- Check the liquid medicine in the syringe before you give your injection. The liquid in the syringe should look clear, and colorless, and may look slightly yellow. If the liquid is cloudy or contains any particles, do not use the syringe and throw it away in a sharps disposal container. See Step 13 below, “Dispose of your needles and syringes.”
Step 4: Choose your injection area. See Figure C.
See the injection areas you should use on your body. Talk with your doctor about the injection areas that are best for you.
- The possible injection areas on your body include (See Figure C):
- your stomach area (abdomen) around the belly button
- the back of your upper arms
- upper hips (below your waist)
- your thighs (above your knees)
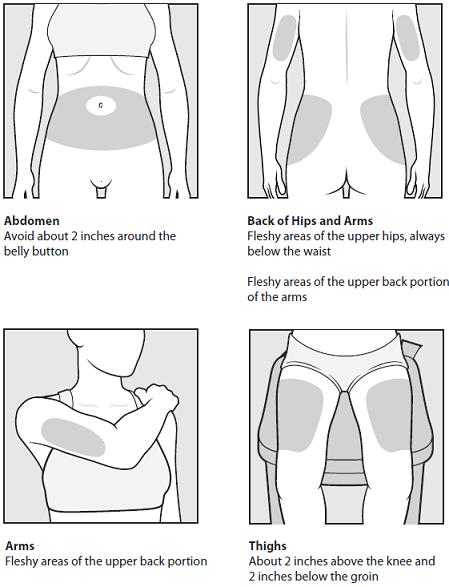
Figure C
- For each COPAXONE dose, choose a different injection area from 1 of the areas shown above. See Figure C.
- Do not stick the needle in the same place (site) more than 1 time each week. Each injection area contains multiple injection sites for you to choose from. Avoid injecting in the same site over and over again.
- Keep a record of the sites where you give your injection each day so you will remember where you already injected.
Step 5: Prepare to give your injection.
- There are some injection areas on your body that are hard to reach (like the back of your arm). You may need help from someone who has been instructed on how to give your injection if you cannot reach certain injection areas.
- Do not inject in sites where the skin has scarring or “dents”. Using scarred or dented skin for your injections may make your skin worse.
Step 6: Clean your injection site.
- Clean the injection site using the alcohol wipe and allow your skin to air dry. See Figure D.
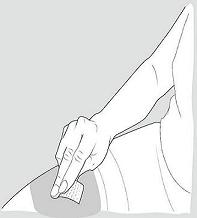
Figure D
Step 7: Pick up the syringe with 1 hand and hold it like a pencil. Remove the needle cover with your other hand and set it aside. See Figure E.
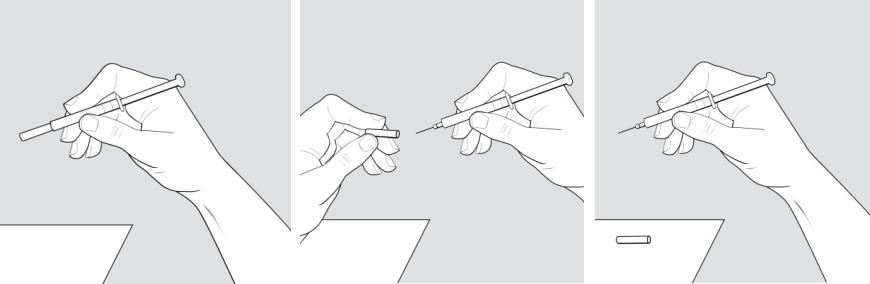
Figure E
Step 8: Pinch about a 2 inch fold of skin between your thumb and index finger. See Figure F.
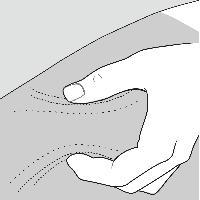
Figure F
Step 9: Giving your injection.
- Rest the heel of your hand holding the syringe against your skin at the injection site. Insert the needle at a 90 degree angle straight into your skin. See Figure G.
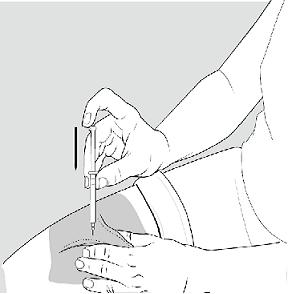
Figure G
- When the needle is all the way into your skin, release the fold of skin. See Figure H.
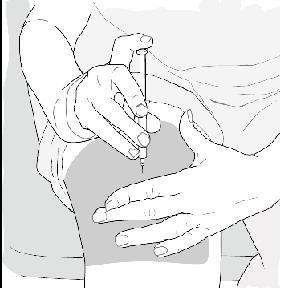
Figure H
Step 10: Give your COPAXONE injection.
To inject the medicine, hold the syringe steady and slowly push down the plunger. See Figure I.
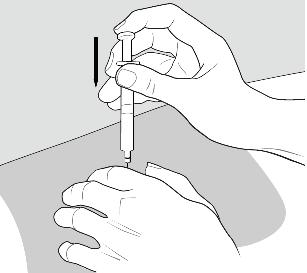
Figure I
Step 11: Remove the needle.
After you have injected all of the medicine, pull the needle straight out. See Figure J.
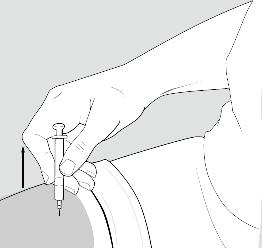
Figure J
Step 12: Use a clean, dry cotton ball to gently press on the injection site for a few seconds. Do not rub the injection site or re-use the needle or syringe. See Figure K.
Figure K
Step 13: Dispose of your needles and syringes.
- Put your used needles and syringes in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and syringes in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
Figure L
This Patient Information and Instructions for Use has been approved by the U.S. Food and Drug Administration.

Marketed by: Teva Neuroscience, Inc., Parsippany, NJ 07054
Distributed by: Teva Pharmaceuticals USA, Inc., Parsippany, NJ 07054©2019 Teva Neuroscience, Inc.
COPIFU-004
Revised: December 2019
- Package/Label Display Panel, Part 1 of 2
-
Package/Label Display Panel, Part 2 of 2

Copaxone® (glatiramer acetate injection) 20 mg/mL, 30 Single-Dose Pre-Filled Syringe Carton Text
NDC: 68546-317-30
30 Single-Dose Pre-Filled Syringes
Each pre-filled syringe contains 1 mL COPAXONE® solution of: glatiramer acetate 20 mg; mannitol 40 mg (inactive ingredient)
ONCE DAILY COPAXONE® (glatiramer acetate injection) 20 mg/mL
Rx Only
FOR SUBCUTANEOUS INJECTION ONLY
TEVA Neuroscience
Marketed by: TEVA Neuroscience, Inc., Overland Park, KS 66211
Distributed by: TEVA Pharmaceuticals USA, Inc., North Wales, PA 19454
Product of Israel - Package/Label Display Panel, Part 1 of 2
-
Package/Label Display Panel, Part 2 of 2

Copaxone® (glatiramer acetate injection) 40 mg/mL, 12 Single-Dose Pre-Filled Syringe Carton Text
NDC: 68546-325-12
12 Single-Dose Pre-Filled Syringes
Each pre-filled syringe contains 1 mL COPAXONE® solution of: glatiramer acetate 40 mg; mannitol 40 mg (inactive ingredient)
THREE TIMES A WEEK COPAXONE® (glatiramer acetate injection)
40 mg/mL
Rx Only
FOR SUBCUTANEOUS INJECTION ONLY
TEVA Neuroscience
Marketed by: TEVA Neuroscience, Inc., Overland Park, KS 66211
Distributed by: TEVA Pharmaceuticals USA, Inc., North Wales, PA 19454
Product of Israel -
INGREDIENTS AND APPEARANCE
COPAXONE
glatiramer acetate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68546-317 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLATIRAMER ACETATE (UNII: 5M691HL4BO) (GLATIRAMER - UNII:U782C039QP) GLATIRAMER ACETATE 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) 40 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68546-317-00 14 in 1 CARTON 06/04/2013 02/28/2015 1 1 in 1 BLISTER PACK 1 1 mL in 1 SYRINGE, GLASS; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC: 68546-317-30 30 in 1 CARTON 04/28/2008 2 1 in 1 BLISTER PACK 2 1 mL in 1 SYRINGE, GLASS; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020622 04/28/2008 COPAXONE
glatiramer acetate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68546-325 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLATIRAMER ACETATE (UNII: 5M691HL4BO) (GLATIRAMER - UNII:U782C039QP) GLATIRAMER ACETATE 40 mg in 1 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) 40 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68546-325-06 6 in 1 CARTON 01/29/2014 1 1 in 1 BLISTER PACK 1 1 mL in 1 SYRINGE, GLASS; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC: 68546-325-12 12 in 1 CARTON 01/29/2014 2 1 in 1 BLISTER PACK 2 1 mL in 1 SYRINGE, GLASS; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020622 01/29/2014 Labeler - Teva Neuroscience, Inc. (009906397)
Trademark Results [Copaxone]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 COPAXONE 74348964 1816603 Live/Registered |
TEVA PHARMACEUTICAL INDUSTRIES LTD. 1993-01-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.