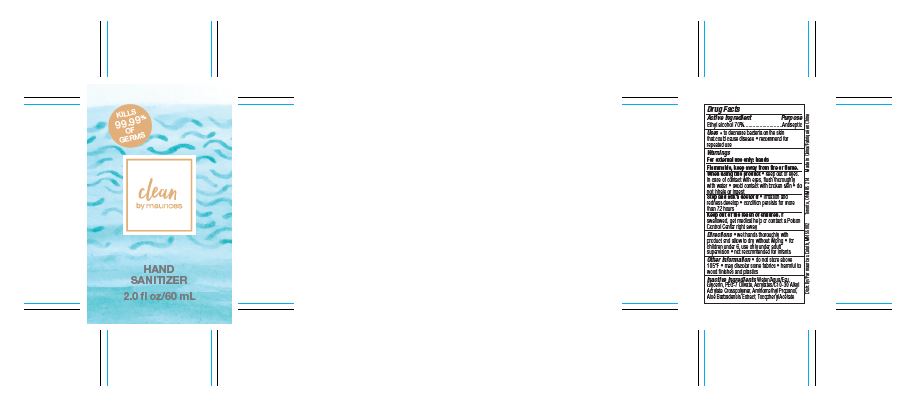
Maurices Clean Hand Sanitizer by Shanghai Sibei Cosmetic Co., Ltd.
Maurices Clean Hand Sanitizer by
Drug Labeling and Warnings
Maurices Clean Hand Sanitizer by is a Otc medication manufactured, distributed, or labeled by Shanghai Sibei Cosmetic Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
MAURICES CLEAN HAND SANITIZER- hand sanitizer gel
Shanghai Sibei Cosmetic Co., Ltd.
----------
Warnings
For external use only: hands
Flammable, keep away from fire or flame.
When using this product
- keep out of eyes. In case of contact with eyes, flush thoroughly with water
- avoid contact with broken skin
- do not inhale or ingest
Directions
- wet hands thoroughly with product and allow to dry without wiping
- for children under 6, use only under adult supervision
- not recommended for infants
Other Information
- do not store above 105ºF
- may discolor some fabrics
- harmful to wood finishes and plastics
| MAURICES CLEAN HAND SANITIZER
hand sanitizer gel |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Shanghai Sibei Cosmetic Co., Ltd. (542969967) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Shanghai Sibei Cosmetic Co., Ltd. | 542969967 | manufacture(79329-001) | |
Revised: 11/2025
Document Id: 43c69d0a-ddbb-6327-e063-6394a90a7894
Set id: aa8ef6e3-798c-e3cc-e053-2a95a90ace0d
Version: 3
Effective Time: 20251117