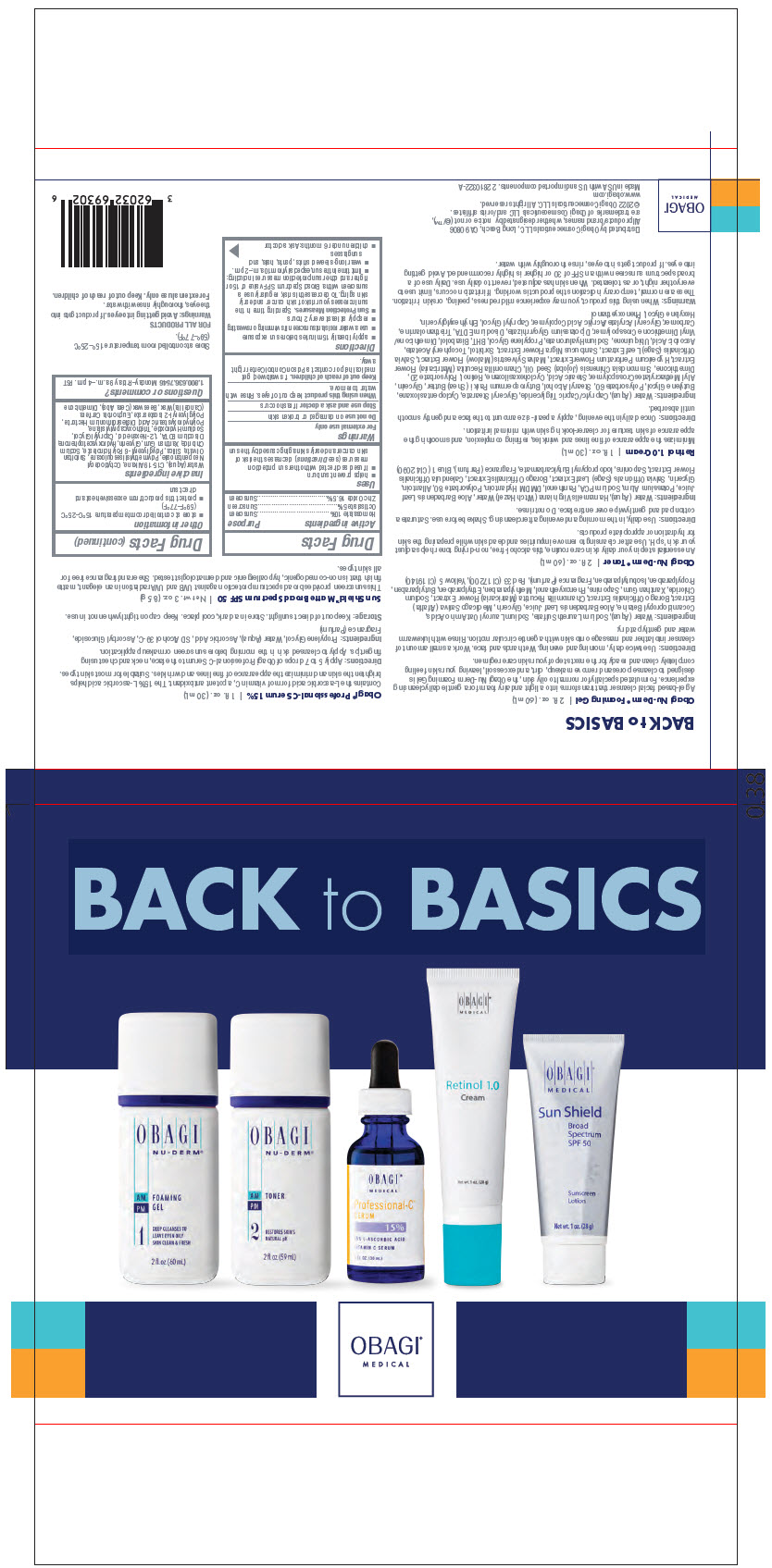
OBAGI MEDICAL BACK TO BASICS (INCLUDING OBAGI MEDICAL PROFESSIONAL-C SERUM 15)- homosalate, octisalate, and zinc oxide kit
Obagi Medical Back to Basics (Including Obagi Medical Professional-C Serum 15) by
Drug Labeling and Warnings
Obagi Medical Back to Basics (Including Obagi Medical Professional-C Serum 15) by is a Otc medication manufactured, distributed, or labeled by Obagi Cosmeceuticals LLC, G.S.COSMECEUTICAL USA, INC., Denison Pharmaceuticals, LLC, Swiss-American CDMO, LLC, TENCO ASSEMBLIES, INC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- helps prevent sunburn
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m.–2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- children under 6 months: Ask a doctor
- Other information
-
Inactive ingredients
Water (Aqua), C15-19 Alkane, Octyldodecyl Neopentanoate, Polymethylsilsesquioxane, Sorbitan Olivate, Silica, Polyglyceryl-6 Polyricinoleate, Sodium Chloride, Xanthan Gum, Glycerin, Hydroxyacetophenone, Disodium EDTA, 1,2-Hexanediol, Caprylyl Glycol, Sodium Hydroxide, Triethoxycaprylylsilane, Polyhydroxystearic Acid, Disteardimonium Hectorite, Polyglyceryl-2 Isostearate, Euphorbia Cerifera (Candelilla) Wax, Beeswax (Cera Alba), Dimethicone
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - Kit Carton
-
INGREDIENTS AND APPEARANCE
OBAGI MEDICAL BACK TO BASICS (INCLUDING OBAGI MEDICAL PROFESSIONAL-C SERUM 15)
homosalate, octisalate, and zinc oxide kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 62032-694 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62032-694-02 1 in 1 CARTON 04/01/2022 1 1 in 1 BAG Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE, PLASTIC 60 mL Part 2 1 BOTTLE, PLASTIC 59 mL Part 3 1 BOTTLE, DROPPER 30 mL Part 4 1 TUBE 30 mL Part 5 1 TUBE 28 g Part 1 of 5 OBAGI NU-DERM FOAMING
cleansing (cold creams, cleansing lotions, liquids, and pads) [skin care preparations (creams, lotions, powder, and sprays)] gelProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) INGR ACETYL HEXAMETHYL TETRALIN (UNII: 360Q8Z01IP) INGR MEDICAGO SATIVA WHOLE (UNII: DJO934BRBD) INGR ALOE VERA LEAF (UNII: ZY81Z83H0X) INGR BORAGO OFFICINALIS SEED (UNII: 2GXJ790US0) INGR BUTYLPARABEN (UNII: 3QPI1U3FV8) INGR BUTYLPHENYL METHYLPROPIONAL (UNII: T7540GJV69) INGR CHAMOMILE (UNII: FGL3685T2X) INGR COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) INGR D&C RED NO. 33 (UNII: 9DBA0SBB0L) INGR DIHYDROMYRCENOL (UNII: 46L1B02ND9) INGR DIOSCOREA VILLOSA TUBER (UNII: IWY3IWX2G8) INGR ETHYLPARABEN (UNII: 14255EXE39) INGR FD&C YELLOW NO. 5 (UNII: I753WB2F1M) INGR GERANYL ACETATE (UNII: 3W81YG7P9R) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR HEXYL SALICYLATE (UNII: 8F78EY72YL) INGR HYDROXYISOHEXYL 3-CYCLOHEXENE CARBOXALDEHYDE (UNII: QUE43B9Z2Q) INGR ISOBUTYLPARABEN (UNII: 0QQJ25X58G) INGR ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) INGR LIMONENE, (+)- (UNII: GFD7C86Q1W) INGR LINALOOL, (+/-)- (UNII: D81QY6I88E) INGR METHYLPARABEN (UNII: A2I8C7HI9T) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR PHENYLETHYL ALCOHOL (UNII: ML9LGA7468) INGR PROPYLPARABEN (UNII: Z8IX2SC1OH) INGR QUILLAJA SAPONARIA BARK (UNII: 8N0K3807ZW) INGR SMILAX ARISTOLOCHIIFOLIA ROOT (UNII: NR100Y25G0) INGR SODIUM CHLORIDE (UNII: 451W47IQ8X) INGR SODIUM LAURETH-3 SULFATE (UNII: BPV390UAP0) INGR SODIUM LAUROYL OAT AMINO ACIDS (UNII: FSW2K9B9N5) INGR WATER (UNII: 059QF0KO0R) INGR XANTHAN GUM (UNII: TTV12P4NEE) INGR YUCCA SCHIDIGERA ROOT (UNII: E2H9ET15AT) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 60 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date COSMETIC 04/01/2022 Part 2 of 5 OBAGI NU-DERM TONER
face and neck (excluding shaving preparations), leave-on [skin care preparations (creams, lotions, powder, and sprays)] liquidProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) INGR ACETYL HEXAMETHYL TETRALIN (UNII: 360Q8Z01IP) INGR ALLANTOIN (UNII: 344S277G0Z) INGR ALOE VERA LEAF (UNII: ZY81Z83H0X) INGR BORAGO OFFICINALIS SEED (UNII: 2GXJ790US0) INGR BUTYLPHENYL METHYLPROPIONAL (UNII: T7540GJV69) INGR CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) INGR DIHYDROMYRCENOL (UNII: 46L1B02ND9) INGR DIOSCOREA VILLOSA TUBER (UNII: IWY3IWX2G8) INGR DMDM HYDANTOIN (UNII: BYR0546TOW) INGR FD&C BLUE NO. 1 (UNII: H3R47K3TBD) INGR GERANYL ACETATE (UNII: 3W81YG7P9R) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR HAMAMELIS VIRGINIANA TOP WATER (UNII: NT00Y05A2V) INGR HEXYL SALICYLATE (UNII: 8F78EY72YL) INGR HYDROXYISOHEXYL 3-CYCLOHEXENE CARBOXALDEHYDE (UNII: QUE43B9Z2Q) INGR IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) INGR ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) INGR LIMONENE, (+)- (UNII: GFD7C86Q1W) INGR LINALOOL, (+/-)- (UNII: D81QY6I88E) INGR PANTHENOL (UNII: WV9CM0O67Z) INGR PHENYLETHYL ALCOHOL (UNII: ML9LGA7468) INGR POLYSORBATE 80 (UNII: 6OZP39ZG8H) INGR POTASSIUM ALUM (UNII: 1L24V9R23S) INGR QUILLAJA SAPONARIA BARK (UNII: 8N0K3807ZW) INGR SAGE (UNII: 065C5D077J) INGR SMILAX ARISTOLOCHIIFOLIA ROOT (UNII: NR100Y25G0) INGR SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) INGR WATER (UNII: 059QF0KO0R) INGR YUCCA SCHIDIGERA ROOT (UNII: E2H9ET15AT) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 59 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date COSMETIC 04/01/2022 Part 3 of 5 OBAGI MEDICAL PROFESSIONAL-C SERUM 15
face and neck (excluding shaving preparations), leave-on [skin care preparations (creams, lotions, powder, and sprays)] liquidProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR ALCOHOL (UNII: 3K9958V90M) INGR ASCORBIC ACID (UNII: PQ6CK8PD0R) INGR ASCORBYL GLUCOSIDE (UNII: 2V52R0NHXW) INGR EVERNIA PRUNASTRI (UNII: O3034Q5AHK) INGR GRAPEFRUIT PEEL (UNII: 3582N05Q44) INGR OCTOXYNOL-9 (UNII: 7JPC6Y25QS) INGR PROPYLENE GLYCOL (UNII: 6DC9Q167V3) INGR PSEUDEVERNIA FURFURACEA (UNII: 49ZMN09Q0K) INGR WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 CARTON 1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date COSMETIC 04/01/2022 Part 4 of 5 OBAGI MEDICAL RETINOL
face and neck (excluding shaving preparations), leave-on [skin care preparations (creams, lotions, powder, and sprays)] creamProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) INGR ALLYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: B9J55EA6QX) INGR ASCORBIC ACID (UNII: PQ6CK8PD0R) INGR BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) INGR BUTYLENE GLYCOL (UNII: 3XUS85K0RA) INGR CAPRYLYL GLYCOL (UNII: 00YIU5438U) INGR CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) INGR CHAMOMILE (UNII: FGL3685T2X) INGR CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) INGR CYCLOMETHICONE 6 (UNII: XHK3U310BA) INGR DIMETHICONE (UNII: 92RU3N3Y1O) INGR DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) INGR EDETATE DISODIUM (UNII: 7FLD91C86K) INGR ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) INGR GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) INGR HEXYLENE GLYCOL (UNII: KEH0A3F75J) INGR HYALURONATE SODIUM (UNII: YSE9PPT4TH) INGR HYPERICUM PERFORATUM FLOWER (UNII: A6V4CUE7PV) INGR JOJOBA OIL (UNII: 724GKU717M) INGR LEVOMENOL (UNII: 24WE03BX2T) INGR MALVA SYLVESTRIS FLOWER (UNII: 12X9JI52BS) INGR MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR POLYSORBATE 20 (UNII: 7T1F30V5YH) INGR POLYSORBATE 60 (UNII: CAL22UVI4M) INGR PROPYLENE GLYCOL (UNII: 6DC9Q167V3) INGR RETINOL (UNII: G2SH0XKK91) INGR SAGE (UNII: 065C5D077J) INGR SAMBUCUS NIGRA FLOWER (UNII: 07V4DX094T) INGR SHEA BUTTER (UNII: K49155WL9Y) INGR SORBITOL (UNII: 506T60A25R) INGR STEARIC ACID (UNII: 4ELV7Z65AP) INGR STEARYL ALCOHOL (UNII: 2KR89I4H1Y) INGR TROLAMINE (UNII: 9O3K93S3TK) INGR UBIDECARENONE (UNII: EJ27X76M46) INGR WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 CARTON 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date COSMETIC 04/01/2022 Part 5 of 5 OBAGI MEDICAL SUN SHIELD BROAD SPECTRUM SPF 50 MATTE SUNCREEN
homosalate, octisalate, and zinc oxide lotionProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 16.5 g in 100 g Inactive Ingredients Ingredient Name Strength 1,2-HEXANEDIOL (UNII: TR046Y3K1G) C15-19 ALKANE (UNII: CI87N1IM01) CANDELILLA WAX (UNII: WL0328HX19) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE (UNII: 92RU3N3Y1O) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) GLYCERIN (UNII: PDC6A3C0OX) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) POLYGLYCERYL-2 ISOSTEARATE (UNII: 7B8OE71MQC) POLYGLYCERYL-6 POLYRICINOLEATE (UNII: YPM0ZOC2HR) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM HYDROXIDE (UNII: 55X04QC32I) SORBITAN OLIVATE (UNII: MDL271E3GR) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) YELLOW WAX (UNII: 2ZA36H0S2V) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 28 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH DRUG M020 07/15/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH DRUG M020 04/01/2022 Labeler - Obagi Cosmeceuticals LLC (790553353) Establishment Name Address ID/FEI Business Operations G.S.COSMECEUTICAL USA, INC. 017014734 MANUFACTURE(62032-694) Establishment Name Address ID/FEI Business Operations Denison Pharmaceuticals, LLC 001207208 MANUFACTURE(62032-694) Establishment Name Address ID/FEI Business Operations Swiss-American CDMO, LLC 080170933 MANUFACTURE(62032-694) Establishment Name Address ID/FEI Business Operations TENCO ASSEMBLIES, INC 075535609 PACK(62032-694)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.