PROPOXYPHENE NAPSYLATE AND ACETAMINOPHEN tablet
PROPOXYPHENE NAPSYLATE AND ACETAMINOPHEN by
Drug Labeling and Warnings
PROPOXYPHENE NAPSYLATE AND ACETAMINOPHEN by is a Prescription medication manufactured, distributed, or labeled by RedPharm Drug Inc., Malinckrodt Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
Propoxyphene napsylate USP is an odorless, white crystalline powder with a bitter taste. It is very slightly soluble in water and soluble in methanol, ethanol, chloroform, and acetone. Chemically, it is (αS,1R)-α-[2-(Dimethylamino)-1-methylethyl]-α-phenylphenethyl propionate compound with 2-naphthalenesulfonic acid (1:1) monohydrate, which can be represented by the accompanying structural formula:
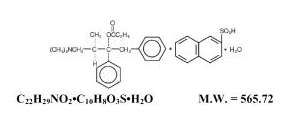
Propoxyphene napsylate differs from propoxyphene hydrochloride in that it allows more stable liquid dosage forms and tablet formulations. Because of differences in molecular weight, a dose of 100 mg (176.8 μmol) of propoxyphene napsylate is required to supply an amount of propoxyphene equivalent to that present in 65 mg (172.9 μmol) of propoxyphene hydrochloride.
The acetaminophen component is 4′-Hydroxyacetanilide, a white, odorless, crystalline powder possessing a slightly bitter taste, and is represented by the following structural formula: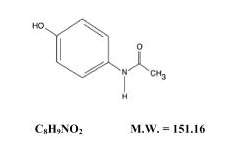
Each tablet of Propoxyphene Napsylate and Acetaminophen Tablets USP, for oral administration, contains 100 mg (176.8 μmol) propoxyphene napsylate and 650 mg (4,300 μmol) acetaminophen.
Each pink tablet also contains crospovidone, D and C Red No. 27 Aluminum Lake, FD and C Yellow No. 6 Aluminum Lake, hydroxypropyl methylcellulose, magnesium stearate, microcrystalline cellulose, polydextrose, polyethylene glycol, povidone, pregelatinized starch, silicon dioxide, stearic acid, titanium dioxide, and triacetin.
Each white tablet also contains crospovidone, hydroxypropyl methylcellulose, magnesium stearate, microcrystalline cellulose, polydextrose, polyethylene glycol, povidone, pregelatinized starch, silicon dioxide, stearic acid, titanium dioxide, and triacetin.
-
CLINICAL PHARMACOLOGY
Propoxyphene is a centrally acting narcotic analgesic agent. Equimolar doses of propoxyphene hydrochloride or napsylate provide similar plasma concentrations. Following administration of 65, 130, or 195 mg of propoxyphene hydrochloride, the bioavailability of propoxyphene is equivalent to that of 100, 200, or 300 mg respectively of propoxyphene napsylate. Peak plasma concentrations of propoxyphene are reached in 2 to 2 1/2 hours. After a 100 mg oral dose of propoxyphene napsylate, peak plasma levels of 0.05 to 0.1 mcg/mL are achieved. As shown in Figure 1, the napsylate salt tends to be absorbed more slowly than the hydrochloride. At or near therapeutic doses, this absorption difference is small when compared with that among subjects and among doses.
Figure 1. Mean plasma concentrations of propoxyphene in 8 human subjects following oral administration of 65 and 130 mg of the hydrochloride salt and 100 and 200 mg of the napsylate salt and in 7 given 195 mg of the hydrochloride and 300 mg of the napsylate salt.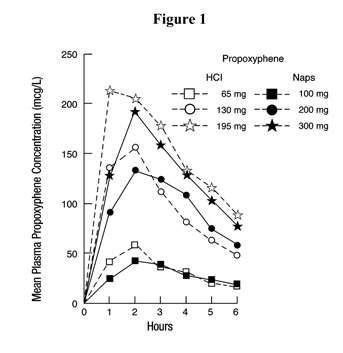
Because of this several hundredfold difference in solubility, the absorption rate of very large doses of the napsylate salt is significantly lower than that of equimolar doses of the hydrochloride.
Repeated doses of propoxyphene at 6-hour intervals lead to increasing plasma concentrations, with a plateau after the ninth dose at 48 hours.
Propoxyphene is metabolized in the liver to yield norpropoxyphene. Propoxyphene has a half-life of 6 to 12 hours, whereas that of norpropoxyphene is 30 to 36 hours.
Norpropoxyphene has substantially less central-nervous-system-depressant effect than propoxyphene but a greater local anesthetic effect, which is similar to that of amitriptyline and antiarrhythmic agents, such as lidocaine and quinidine.
In animal studies in which propoxyphene and norpropoxyphene were continuously infused in large amounts, intracardiac conduction time (PR and QRS intervals) was prolonged. Any intracardiac conduction delay attributable to high concentrations of norpropoxyphene may be of relatively long duration.
ActionsPropoxyphene is a mild narcotic analgesic structurally related to methadone. The potency of propoxyphene napsylate is from two thirds to equal that of codeine.
Propoxyphene napsylate and acetaminophen tablets provide the analgesic activity of propoxyphene napsylate and the antipyretic-analgesic activity of acetaminophen.
The combination of propoxyphene and acetaminophen produces greater analgesia than that produced by either propoxyphene or acetaminophen administered alone.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
- Do not prescribe propoxyphene for patients who are suicidal or addiction-prone.
- Prescribe propoxyphene with caution for patients taking tranquilizers or antidepressant drugs and patients who use alcohol in excess.
- Tell your patients not to exceed the recommended dose and to limit their intake of alcohol.
Propoxyphene products in excessive doses, either alone or in combination with other CNS depressants, including alcohol, are a major cause of drug-related deaths. Fatalities within the first hour of overdosage are not uncommon. In a survey of deaths due to overdosage conducted in 1975, in approximately 20% of the fatal cases, death occurred within the first hour (5% occurred within 15 minutes). Propoxyphene should not be taken in doses higher than those recommended by the physician. The judicious prescribing of propoxyphene is essential to the safe use of this drug. With patients who are depressed or suicidal, consideration should be given to the use of non-narcotic analgesics. Patients should be cautioned about the concomitant use of propoxyphene products and alcohol because of potentially serious CNS-additive effects of these agents. Because of its added depressant effects, propoxyphene should be prescribed with caution for those patients whose medical condition requires the concomitant administration of sedatives, tranquilizers, muscle relaxants, antidepressants, or other CNS-depressant drugs. Patients should be advised of the additive depressant effects of these combinations.
Usage in Ambulatory Patients
Many of the propoxyphene-related deaths have occurred in patients with previous histories of emotional disturbances or suicidal ideation or attempts as well as histories of misuse of tranquilizers, alcohol, and other CNS-active drugs. Some deaths have occurred as a consequence of the accidental ingestion of excessive quantities of propoxyphene alone or in combination with other drugs. Patients taking propoxyphene should be warned not to exceed the dosage recommended by the physician.Propoxyphene may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks, such as driving a car or operating machinery. The patient should be cautioned accordingly.
- PRECAUTIONS
-
Information for Patients
See accompanying Patient Information Sheet.
-
Drug Interactions
The CNS-depressant effect of propoxyphene is additive with that of other CNS depressants, including alcohol.
As is the case with many medicinal agents, propoxyphene may slow the metabolism of a concomitantly administered drug. Should this occur, the higher serum concentrations of that drug may result in increased pharmacologic or adverse effects of that drug. Such occurrences have been reported when propoxyphene was administered to patients on antidepressants, anticonvulsants, or warfarin-like drugs. Severe neurologic signs, including coma, have occurred with concurrent use of carbamazepine. -
Pregnancy
Safe use in pregnancy has not been established relative to possible adverse effects on fetal development. Instances of withdrawal symptoms in the neonate have been reported following usage during pregnancy. Therefore, propoxyphene should not be used in pregnant women unless, in the judgment of the physician, the potential benefits outweigh the possible hazards.
- Nursing Mothers
- Pediatric Use
- Geriatric Use
-
ADVERSE REACTIONS
In a survey conducted in hospitalized patients, less than 1% of patients taking propoxyphene hydrochloride at recommended doses experienced side effects. The most frequently reported were dizziness, sedation, nausea, and vomiting. Some of these adverse reactions may be alleviated if the patient lies down.
Other adverse reactions include constipation, abdominal pain, skin rashes, lightheadedness, headache, weakness, euphoria, dysphoria, hallucinations, and minor visual disturbances.
Liver dysfunction has been reported in association with both active components of propoxyphene napsylate and acetaminophen tablets. Propoxyphene therapy has been associated with abnormal liver function tests and, more rarely, with instances of reversible jaundice (including cholestatic jaundice). Hepatic necrosis may result from acute overdose of acetaminophen (see OVERDOSAGE). In chronic ethanol abusers, this has been reported rarely with short-term use of acetaminophen dosages of 2.5 to 10 g/day. Fatalities have occurred.
Renal papillary necrosis may result from chronic acetaminophen use, particularly when the dosage is greater than recommended and when combined with aspirin.
Subacute painful myopathy has occurred following chronic propoxyphene overdosage. -
DRUG ABUSE AND DEPENDENCE
Propoxyphene, when taken in higher-than-recommended doses over long periods of time, can produce drug dependence characterized by psychic dependence and, less frequently, physical dependence and tolerance. Propoxyphene will only partially suppress the withdrawal syndrome in individuals physically dependent on morphine or other narcotics. The abuse liability of propoxyphene is qualitatively similar to that of codeine although quantitatively less, and propoxyphene should be prescribed with the same degree of caution appropriate to the use of codeine.
-
OVERDOSAGE
In all cases of suspected overdosage, call your regional Poison Control Center to obtain the most up-to-date information about the treatment of overdose. This recommendation is made because, in general, information regarding the treatment of overdosage may change more rapidly than do package inserts.
Symptoms of Propoxyphene Overdosage
Initial consideration should be given to the management of the CNS effects of propoxyphene overdosage. Resuscitative measures should be initiated promptly.The manifestations of acute overdosage with propoxyphene are those of narcotic overdosage. The patient is usually somnolent but may be stuporous or comatose and convulsing. Respiratory depression is characteristic. The ventilatory rate and/or tidal volume is decreased, which results in cyanosis and hypoxia. Pupils, initially pinpoint, may become dilated as hypoxia increases. Cheyne-Stokes respiration and apnea may occur. Blood pressure and heart rate are usually normal initially, but blood pressure falls and cardiac performance deteriorates, which ultimately results in pulmonary edema and circulatory collapse, unless the respiratory depression is corrected and adequate ventilation is restored promptly. Cardiac arrhythmias and conduction delay may be present. A combined respiratory-metabolic acidosis occurs owing to retained CO2 (hypercapnia) and to lactic acid formed during anaerobic glycolysis. Acidosis may be severe if large amounts of salicylates have also been ingested. Death may occur.
Treatment of Propoxyphene OverdosageAttention should be directed first to establishing a patent airway and to restoring ventilation. Mechanically assisted ventilation, with or without oxygen, may be required, and positive pressure respiration may be desirable if pulmonary edema is present. The narcotic antagonist naloxone will markedly reduce the degree of respiratory depression, and 0.4 to 2 mg should be administered promptly, preferably intravenously. If the desired degree of counteraction with improvement in respiratory functions is not obtained, naloxone should be repeated at 2- to 3-minute intervals. The duration of action of the antagonist may be brief. If no response is observed after 10 mg of naloxone have been administered, the diagnosis of propoxyphene toxicity should be questioned. Naloxone may also be administered by continuous intravenous infusion.
Treatment of Propoxyphene Overdosage in Pediatric PatientsThe usual initial dose of naloxone in pediatric patients is 0.01 mg/kg body weight given intravenously. If this dose does not result in the desired degree of clinical improvement, a subsequent increased dose of 0.1 mg/kg body weight may be administered. If an IV route of administration is not available, naloxone may be administered IM or subcutaneously in divided doses. If necessary, naloxone can be diluted with Sterile Water for Injection.
Symptoms of Acetaminophen Overdosage
Blood gases, pH, and electrolytes should be monitored in order that acidosis and any electrolyte disturbance present may be corrected promptly. Acidosis, hypoxia, and generalized CNS depression predispose to the development of cardiac arrhythmias. Ventricular fibrillation or cardiac arrest may occur and necessitate the full complement of cardiopulmonary resuscitation (CPR) measures. Respiratory acidosis rapidly subsides as ventilation is restored and hypercapnia eliminated, but lactic acidosis may require intravenous bicarbonate for prompt correction.
Electrocardiographic monitoring is essential. Prompt correction of hypoxia, acidosis, and electrolyte disturbance (when present) will help prevent these cardiac complications and will increase the effectiveness of agents administered to restore normal cardiac function.
In addition to the use of a narcotic antagonist, the patient may require careful titration with an anticonvulsant to control convulsions. Analeptic drugs (for example, caffeine or amphetamine) should not be used because of their tendency to precipitate convulsions.
General supportive measures, in addition to oxygen, include, when necessary, intravenous fluids, vasopressor-inotropic compounds, and, when infection is likely, anti-infective agents. Gastric lavage may be useful, and activated charcoal can adsorb a significant amount of ingested propoxyphene. Dialysis is of little value in poisoning due to propoxyphene. Efforts should be made to determine whether other agents, such as alcohol, barbiturates, tranquilizers, or other CNS depressants, were also ingested, since these increase CNS depression as well as cause specific toxic effects.Shortly after oral ingestion or an overdose of acetaminophen and for the next 24 hours, anorexia, nausea, vomiting, diaphoresis, general malaise, and abdominal pain have been noted. The patient may then present no symptoms, but evidence of liver dysfunction may become apparent up to 72 hours after ingestion, with elevated serum transaminase and lactic dehydrogenase levels, an increase in serum bilirubin concentrations, and a prolonged prothrombin time. Death from hepatic failure may result 3 to 7 days after overdosage.
Treatment of Acetaminophen Overdosage
Acute renal failure may accompany the hepatic dysfunction and has been noted in patients who do not exhibit signs of fulminant hepatic failure. Typically, renal impairment is more apparent 6 to 9 days after ingestion of the overdose.Acetaminophen in massive overdosage may cause hepatic toxicity in some patients. In all cases of suspected overdose, immediately call your regional poison center or the Rocky Mountain Poison Center’s toll-free number (800-525-6115) for assistance in diagnosis and for directions in the use of N-acetylcysteine as an antidote.
In adults, hepatic toxicity has rarely been reported with acute overdoses of less than 10 g and fatalities with less than 15 g. Importantly, young children seem to be more resistant than adults to the hepatotoxic effect of an acetaminophen overdose. Despite this, the measures outlined below should be initiated in any adult or pediatric patients suspected of having ingested an acetaminophen overdose.
Because clinical and laboratory evidence of hepatic toxicity may not be apparent until 48 to 72 hours postingestion, liver function studies should be obtained initially and repeated at 24-hour intervals.
Consider emptying the stomach promptly by lavage or by induction of emesis with syrup of ipecac. Patients’ estimates of the quantity of a drug ingested are notoriously unreliable. Therefore, if an acetaminophen overdose is suspected, a serum acetaminophen assay should be obtained as early as possible, but no sooner than 4 hours following ingestion. The antidote, N-acetylcysteine, should be administered as early as possible, and within 16 hours of the overdose ingestion for optimal results. Following recovery, there are no residual, structural, or functional hepatic abnormalities. -
HOW SUPPLIED
This product is given orally. The usual dosage is 100 mg propoxyphene napsylate and 650 mg acetaminophen every 4 hours as needed for pain. The maximum recommended dose of propoxyphene napsylate is 600 mg per day.
Consideration should be given to a reduced total daily dosage in patients with hepatic or renal impairment. -
HOW SUPPLIED
Each pink Propoxyphene Napsylate and Acetaminophen Tablet USP 100 mg/650 mg is available as a capsule-shaped, pink, coated tablet, one side debossed M and the other side debossed “1772”.
Bottles of 15.......................................NDC: 67296-0105-1
Dispense in tight containers. Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
-
ANIMAL PHARMACOLOGY & OR TOXICOLOGY
The acute lethal doses of the hydrochloride and napsylate salts of propoxyphene were determined in 4 species. The results shown in Figure 2 indicate that, on a molar basis, the napsylate salt is less toxic than the hydrochloride. This may be due to the relative insolubility and retarded absorption of propoxyphene napsylate.
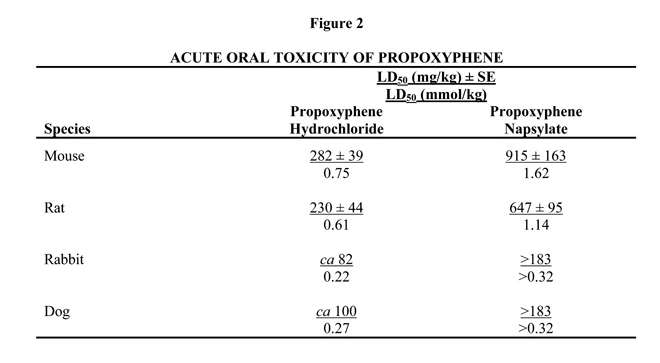
Some indication of the relative insolubility and retarded absorption of propoxyphene napsylate was obtained by measuring plasma propoxyphene levels in 2 groups of 4 dogs following oral administration of equimolar doses of the 2 salts. As shown in Figure 3, the peak plasma concentration observed with propoxyphene hydrochloride was much higher than that obtained after administration of the napsylate salt.
Although none of the animals in this experiment died, 3 of the 4 dogs given propoxyphene hydrochloride exhibited convulsive seizures during the time interval corresponding to the peak plasma levels. The 4 animals receiving the napsylate salt were mildly ataxic but not acutely ill.
Figure 3. Plasma propoxyphene concentrations in dogs following large doses of the hydrochloride and napsylate salts.Mallinckrodt Inc.,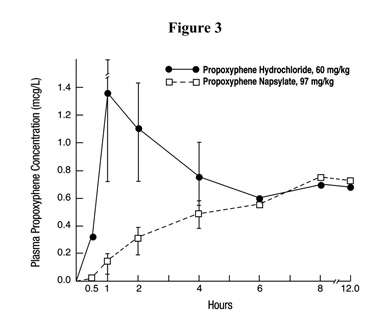
Hazelwood, MO 63042 USA.
Printed in U.S.A.
- Label
-
INGREDIENTS AND APPEARANCE
PROPOXYPHENE NAPSYLATE AND ACETAMINOPHEN
propoxyphene napsylate and acetaminophen tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 67296-0105(NDC: 0406-1772) Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PROPOXYPHENE NAPSYLATE (UNII: 38M219L1OJ) (PROPOXYPHENE - UNII:S2F83W92TK) PROPOXYPHENE NAPSYLATE 100 mg ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 650 mg Inactive Ingredients Ingredient Name Strength CROSPOVIDONE (UNII: 68401960MK) HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYDEXTROSE (UNII: VH2XOU12IE) POLYETHYLENE GLYCOLS (UNII: 3WJQ0SDW1A) POVIDONE (UNII: FZ989GH94E) STARCH, CORN (UNII: O8232NY3SJ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) ALUMINUM OXIDE (UNII: LMI26O6933) Product Characteristics Color pink Score no score Shape OVAL Size 19mm Flavor Imprint Code M;1772 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 67296-0105-1 15 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA075738 06/08/2009 Labeler - RedPharm Drug Inc. (008039641) Establishment Name Address ID/FEI Business Operations Malinckrodt Inc. 957414238 manufacture, analysis
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
