Premium Hand Sanitizer by VITANE PHARMACEUTICALS INC / Welburn Global, Sociedad Anonima
Premium Hand Sanitizer by
Drug Labeling and Warnings
Premium Hand Sanitizer by is a Otc medication manufactured, distributed, or labeled by VITANE PHARMACEUTICALS INC, Welburn Global, Sociedad Anonima. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
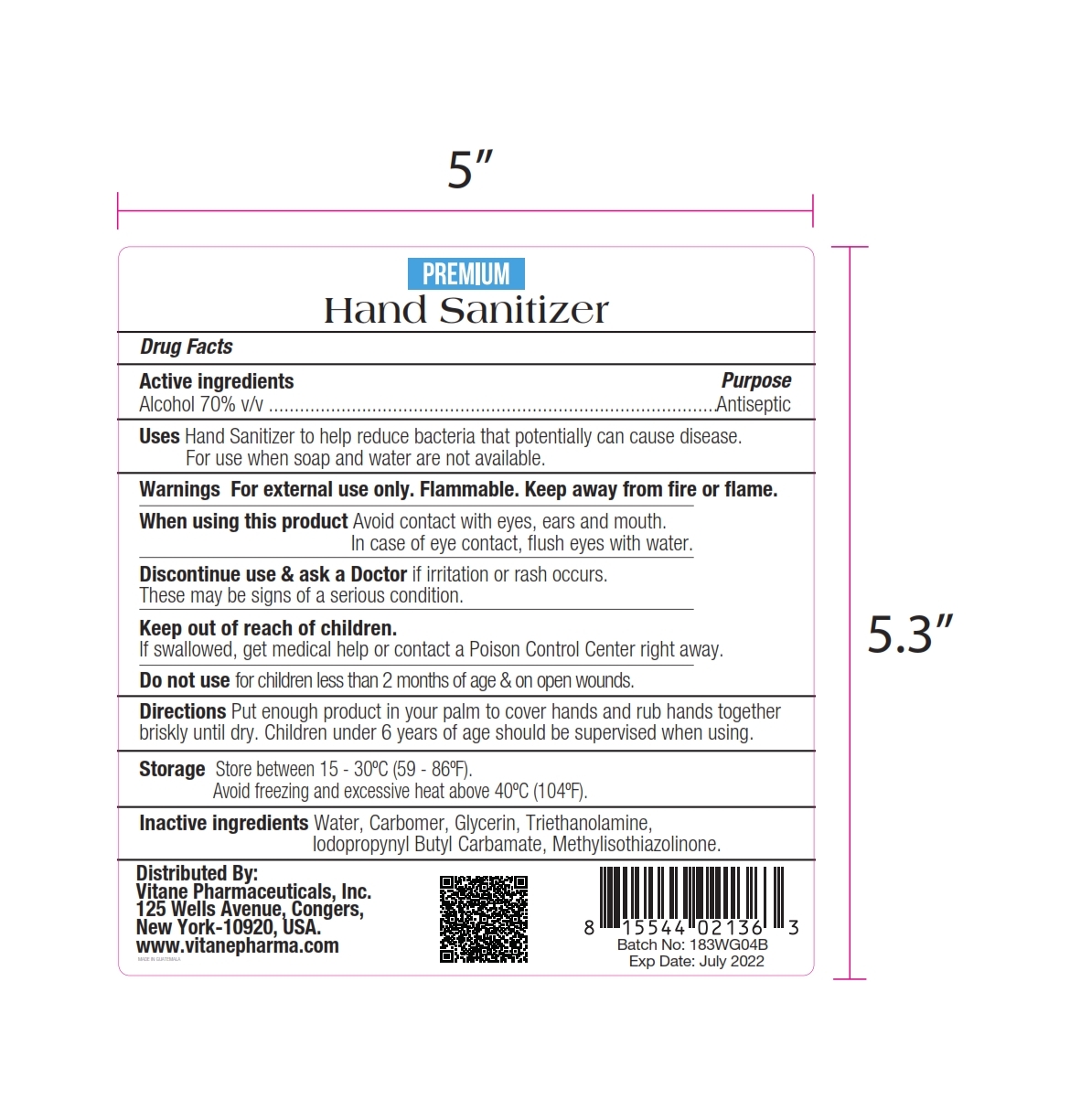
PREMIUM HAND SANITIZER- alcohol gel
VITANE PHARMACEUTICALS INC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
This is a hand sanitizer manufactured according to the Temporary Policy for Preparation of Certain Alcohol-Based Hand Sanitizer Products During the Public Health Emergency (CoViD-19); Guidance for Industry.
The hand sanitizer is manufactured using only the following United States Pharmacopoeia (USP) grade ingredients in the preparation of the product (percentage in final product formulation) consistent with World Health Organization (WHO) recommendations:
- Alcohol (ethanol) (USP or Food Chemical Codex (FCC) grade) (70%, volume/volume (v/v)) in an aqueous solution denatured according to Alcohol and Tobacco Tax and Trade Bureau regulations in 27 CFR part 20.
- Glycerin (0.5% v/v).
- Sterile distilled water or boiled cold water.
Use
Hand Sanitizer to help reduce bacteria that potentially can cause disease. For use when soap and water are not available.
When using this product keep out of eyes, ears, and mouth. In case of contact with eyes, rinse eyes thoroughly with water.
Stop use and ask a doctor if irritation or rash occurs. These may be signs of a serious condition.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Place enough product on hands to cover all surfaces. Rub hands together until dry.
- Supervise children under 6 years of age when using this product to avoid swallowing.
| PREMIUM HAND SANITIZER
alcohol gel |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - VITANE PHARMACEUTICALS INC (029029638) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Welburn Global, Sociedad Anonima | 817579163 | manufacture(60577-774) | |

 3785 mL NDC:
3785 mL NDC: