ALLERGY RELIEF 4 HOUR- chlorpheniramine maleate tablet
Allergy Relief by
Drug Labeling and Warnings
Allergy Relief by is a Otc medication manufactured, distributed, or labeled by Cardinal Health 110, LLC. DBA Leader, LNK International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- trouble urinating due to an enlarged prostate gland
-
Directions
adults and children 12
years of age and over1 tablet every 4 to 6 hours. Do not take more than 6 tablets in 24 hours. children 6 to under 12 years of age 1/2 tablet (break tablet in half) every 4 to 6 hours. Do not exceed 3 whole tablets in 24 hours. children under 6 years of age do not use this product in children under 6 years of age - Other information
- Inactive ingredients
- Questions or comments?
-
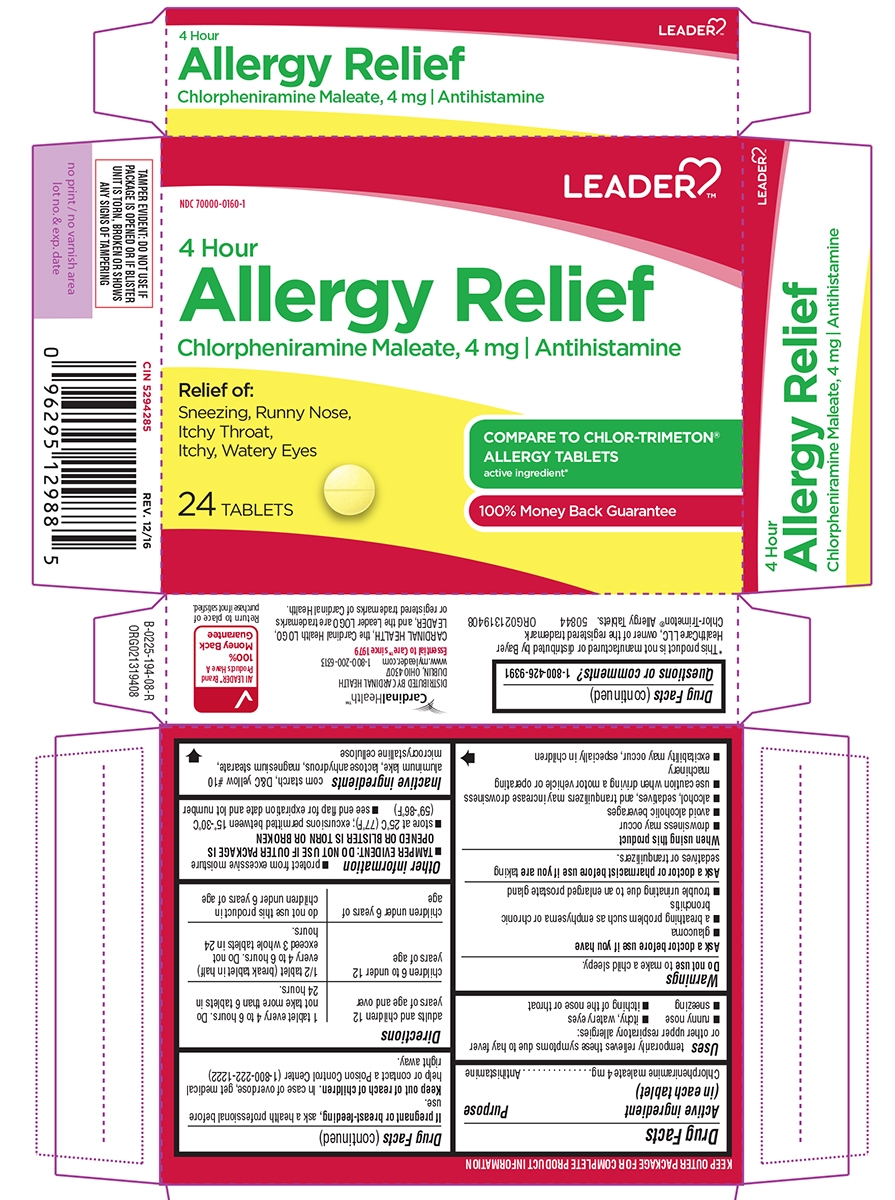
Principal display panel
NDC: 70000-0160-1
LEADER™
4 Hour
Allergy Relief
Chlorpheniramine Maleate, 4 mg ι AntihistamineRelief of:
Sneezing, Runny Nose,
Itchy Throat,
Itchy, Watery Eyes24 TABLETS
COMPARE TO CHLOR-TRIMETON® ALLERGY TABLETS
active ingredient*100% Money Back Guarantee
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING
CIN 5294285 REV. 12/16
*This product is not manufactured or distributed by Bayer HealthCare LLC, owner of the registered trademark Chlor-Trimeton® Allergy Tablets.
50844 ORG021319408
CardinalHealth™
DISTRIBUTED BY CARDINAL HEALTH
DUBLIN, OHIO 43017
www.myleader.com 1-800-200-6313
Essential to Care™ since 1979CARDINAL HEALTH, the Cardinal Health LOGO,
LEADER, and the Leader LOGO are trademarks
or registered trademarks of Cardinal Health.All LEADER™ Brand
Products Have A
100%
Money Back
GuaranteeReturn to place of purchase if not satisfied.
Leader 44-194
-
INGREDIENTS AND APPEARANCE
ALLERGY RELIEF 4 HOUR
chlorpheniramine maleate tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 70000-0160 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORPHENIRAMINE MALEATE (UNII: V1Q0O9OJ9Z) (CHLORPHENIRAMINE - UNII:3U6IO1965U) CHLORPHENIRAMINE MALEATE 4 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) Product Characteristics Color YELLOW Score 2 pieces Shape ROUND Size 8mm Flavor Imprint Code 44;194 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70000-0160-1 2 in 1 CARTON 12/19/1992 1 12 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC: 70000-0160-2 1 in 1 CARTON 12/19/1992 2 100 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part341 12/19/1992 Labeler - Cardinal Health (097537435) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 PACK(70000-0160) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867894 MANUFACTURE(70000-0160) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 PACK(70000-0160) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 PACK(70000-0160) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 868734088 PACK(70000-0160)
Trademark Results [Allergy Relief]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ALLERGY RELIEF 98236984 not registered Live/Pending |
Dmytro Kononenko 2023-10-24 |
 ALLERGY RELIEF 90457167 not registered Live/Pending |
American Textile Company, Inc. 2021-01-10 |
 ALLERGY RELIEF 78838437 3358249 Live/Registered |
Meshbesher Health Corporation 2006-03-16 |
 ALLERGY RELIEF 76619855 3066888 Live/Registered |
AMERICAN TEXTILE COMPANY 2004-11-09 |
 ALLERGY RELIEF 74668018 not registered Dead/Abandoned |
NaturaLife Corporation 1995-05-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.