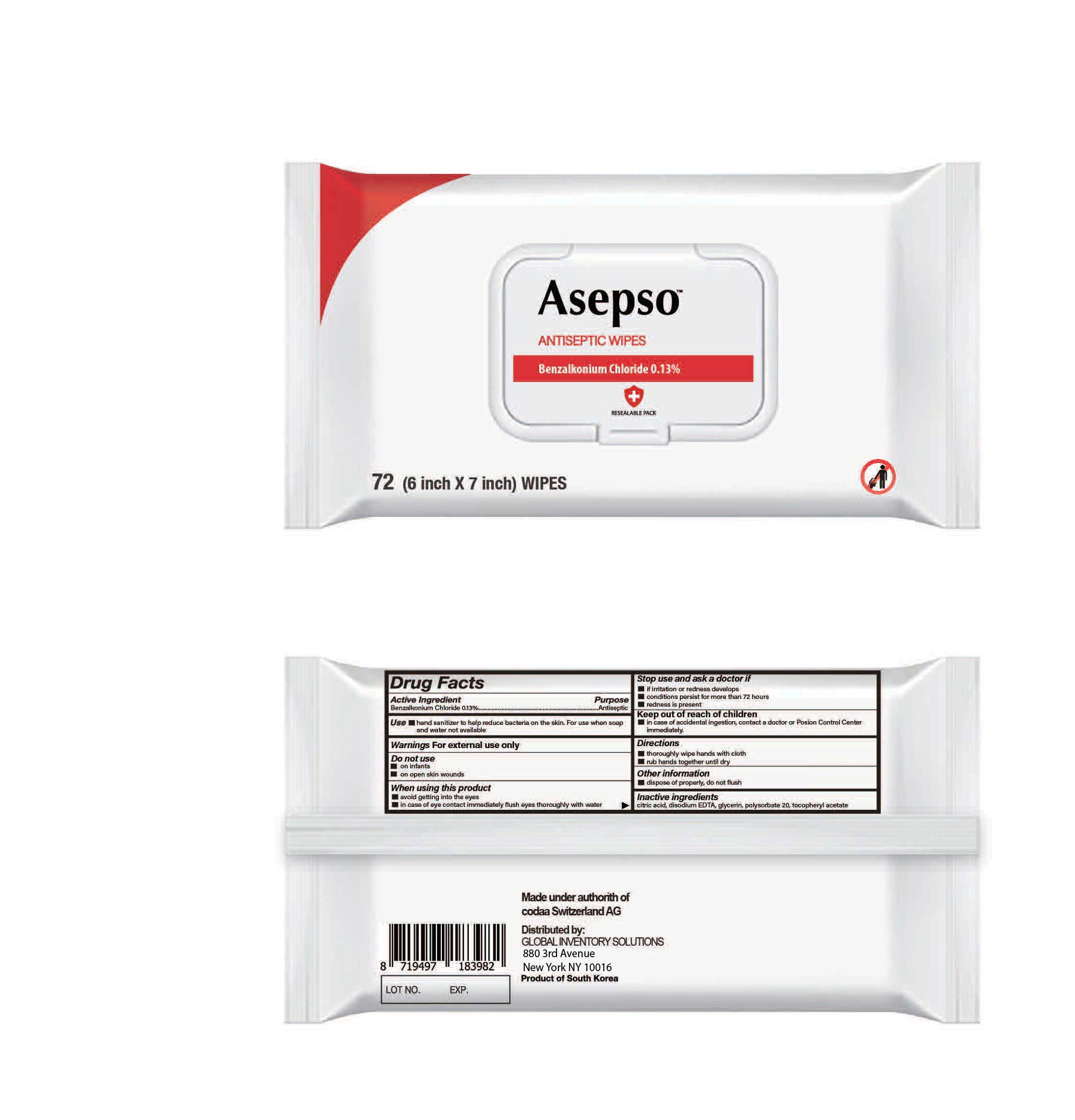
Asepso Wipes BZK0.13Cmplted
Antiseptic Wipes by
Drug Labeling and Warnings
Antiseptic Wipes by is a Otc medication manufactured, distributed, or labeled by GLOBAL INVENTORY SOLUTIONS CORP.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ANTISEPTIC WIPES- benzalkonium chloride cloth
GLOBAL INVENTORY SOLUTIONS CORP.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Asepso Wipes BZK0.13Cmplted
When using this product
■ avoid getting into the eyes
■ in case of eye contact immediately flush eyes thoroughly with water
Stop use and ask a doctor if
■ irritation or redness develops
■ conditions persist for more than 72 hours
■ redness is present
| ANTISEPTIC WIPES
benzalkonium chloride cloth |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - GLOBAL INVENTORY SOLUTIONS CORP. (117535121) |
Revised: 8/2020
Document Id: adb8caa3-a582-6b29-e053-2995a90a4fba
Set id: ab08eb40-e14f-359e-e053-2a95a90a1033
Version: 2
Effective Time: 20200825