TECELRA- afamitresgene autoleucel injection, suspension
TECELRA by
Drug Labeling and Warnings
TECELRA by is a Other medication manufactured, distributed, or labeled by USWM, LLC, Adaptimmune LLC, USWM CT, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TECELRA safely and effectively. See full prescribing information for TECELRA.
TECELRA® (afamitresgene autoleucel) suspension, for intravenous infusion
Initial U.S. Approval: 2024WARNING: CYTOKINE RELEASE SYNDROME
See full prescribing information for complete boxed warning.Cytokine Release Syndrome (CRS), which may be severe or life-threatening, occurred in patients receiving TECELRA. At the first sign of CRS, immediately evaluate patient for hospitalization and institute treatment with supportive care. Ensure that healthcare providers administering TECELRA have immediate access to medications and resuscitative equipment to manage CRS (2.2, 5.1).
INDICATIONS AND USAGE
TECELRA is a melanoma-associated antigen A4 (MAGE-A4)-directed genetically modified autologous T cell immunotherapy indicated for the treatment of adults with unresectable or metastatic synovial sarcoma who have received prior chemotherapy, are HLA-A*02:01P, -A*02:02P, -A*02:03P, or -A*02:06P positive and whose tumor expresses the MAGE-A4 antigen as determined by FDA-approved or cleared companion diagnostic devices.
This indication is approved under accelerated approval based on overall response rate and duration of response (14). Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
DOSAGE AND ADMINISTRATION
For autologous use only. For intravenous use only.
Prior to infusion- Verify patient's identity prior to infusion (2.2).
- Administer a lymphodepleting regimen of cyclophosphamide and fludarabine (2.2).
- Premedicate with acetaminophen and an H1-antihistamine (2.2).
TECELRA Dose and Administration
The recommended dose is between 2.68 x 109 to 10 x 109 MAGE-A4 T cell receptor (TCR) positive T cells (2.1).Administer each infusion bag within one hour of thawing.
DO NOT USE a leukodepleting filter (2.2).
DO NOT USE prophylactic systemic corticosteroids (2.2).DOSAGE FORMS AND STRENGTHS
TECELRA is
- A cell suspension for intravenous infusion.
- Provided in one or more infusion bag(s) containing 2.68 x 109 to 10 x 109 MAGE-A4 TCR positive T cells (3).
CONTRAINDICATIONS
DO NOT use TECELRA in adults who are heterozygous or homozygous for HLA-A*02:05P (4).
WARNINGS AND PRECAUTIONS
Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS): Monitor for ICANS events for at least 4 weeks after treatment with TECELRA (5.2).
Prolonged Severe Cytopenia: Patients may exhibit severe cytopenia (hemoglobin < 8.0 g/dL, neutrophils < 1,000/mm3, platelets < 50,000/mm3) for several weeks following lymphodepleting chemotherapy and TECELRA infusion. Monitor blood counts prior to and after TECELRA infusion (5.3).
Infections: Monitor patients for signs and symptoms of infection; treat appropriately (5.4).
Secondary Malignancies: In the event that a secondary malignancy occurs after treatment with TECELRA, contact 1-855-246-9232 (5.5).
Hypersensitivity Reactions: Monitor for hypersensitivity reactions during infusion (5.6).
Effects on Ability to Drive and Use Machines: Advise patients to refrain from driving and engaging in hazardous occupations or activities, such as operating heavy or potentially dangerous machinery, for at least 4 weeks after receiving TECELRA (5.2).
ADVERSE REACTIONS
Most common adverse reactions (≥ 20%) were, cytokine release syndrome, nausea, vomiting, fatigue, infections, pyrexia, constipation, dyspnea, abdominal pain, non-cardiac chest pain, decreased appetite, tachycardia, back pain, hypotension, diarrhea, and edema.
Grade 3 or 4 laboratory abnormalities (≥20%) were lymphocyte count decreased, neutrophil count decreased, white cell blood count decreased, red blood cell decreased, and platelet count decreased (6.1).
The most common serious adverse reactions (≥ 5%) were cytokine release syndrome and pleural effusion (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact USWM CT, LLC at 1-855-246-9232 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 1/2026
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: CYTOKINE RELEASE SYNDROME
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dose
2.2 Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Cytokine Release Syndrome
5.2 Immune Effector Cell-associated Neurotoxicity Syndrome
5.3 Prolonged Severe Cytopenia
5.4 Infections
5.5 Secondary Malignancies
5.6 Hypersensitivity Reactions
5.7 Potential for HIV Nucleic Acid Test False-Positive Results
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: CYTOKINE RELEASE SYNDROME
Cytokine Release Syndrome (CRS), which may be severe or life-threatening, occurred in patients receiving TECELRA. At the first sign of CRS, immediately evaluate patient for hospitalization and institute treatment with supportive care. Ensure that healthcare providers administering TECELRA have immediate access to medications and resuscitative equipment to manage CRS [see Preparation and Administration (2.2), and Warnings and Precautions (5.1)].
-
1 INDICATIONS AND USAGE
TECELRA is a melanoma-associated antigen A4-(MAGE-A4)-directed genetically modified autologous T cell immunotherapy indicated for the treatment of adults with unresectable or metastatic synovial sarcoma who have received prior chemotherapy, are HLA-A*02:01P, -A*02:02P, -A*02:03P, or -A*02:06P positive and whose tumor expresses the MAGE-A4 antigen as determined by FDA-approved or cleared companion diagnostic devices.
This indication is approved under accelerated approval based on overall response rate and durability of response [see Clinical Studies (14)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
-
2 DOSAGE AND ADMINISTRATION
For autologous use only. For intravenous use only.
2.1 Recommended Dose
The recommended dose is between 2.68 x 109 to 10 x 109 MAGE-A4 T cell receptor (TCR) positive T cells administered as a single intravenous infusion.
TECELRA is provided as a single dose for infusion in one or more infusion bag(s). Verify the number of bags received for the indicated dose prior to preparation for infusion.
2.2 Preparation and Administration
Receipt of TECELRA
Plan for TECELRA to arrive prior to beginning lymphodepleting chemotherapy.
Ensure storage conditions in vapor phase of liquid nitrogen (≤ -130°C).
TECELRA is shipped directly to the healthcare facility in the vapor phase of a liquid nitrogen shipper. Upon receipt of TECELRA confirm the patient's identifiers on the metal cassette and product bag.
Inspect the product for obvious signs of damage and contact 1-855-246-9232 if any anomalies are identified at the time of receipt.
Transfer TECELRA in the original packaging, containing the cassette(s) protecting the infusion bag(s), to onsite storage at ≤ -130°C before the shipper expires.
Store TECELRA in a manner that is consistent with How Supplied/Storage and Handling (16). If unforeseen circumstances prevent proper storage of TECELRA consistent with How Supplied/Storage and Handling (16), contact 1-855-246-9232 to arrange for return shipment.
Preparing Patient for TECELRA Administration
Confirm availability of TECELRA at the healthcare facility prior to starting the lymphodepleting chemotherapy regimen.
Match the patient's identity with the patient identifiers on the TECELRA cassette(s) and infusion bag(s). Do not infuse TECELRA if the information on the patient-specific label(s) does not match the intended patient.
Administer a lymphodepleting chemotherapy regimen of fludarabine 30 mg/m2/day intravenously for 4 days starting on the seventh day before TECELRA infusion (Day-7 to Day -4) and cyclophosphamide 600 mg/m2/day intravenously for 3 days starting the seventh day before TECELRA infusion (Day -7 to Day -5).
Refer to fludarabine prescribing for information on fludarabine dosage in patients with renal impairment.
Short-acting or pegylated granulocyte-colony stimulating factor (G-CSF) may be administered at the discretion of the physician, and according with institutional standards, from 24 hours after last day of lymphodepleting chemotherapy (from Day -3) until resolution of neutropenia.
Premedication
Premedicate with an H1-antihistamine and acetaminophen according to institutional standard practice, approximately 30-60 minutes prior to TECELRA infusion.
Avoid prophylactic systemic corticosteroids, as it may interfere with the activity of TECELRA.
Preparation of TECELRA for Administration
Do not thaw the product until it is ready to be used. Coordinate the timing of TECELRA thaw and infusion. Confirm infusion time in advance and adjust the start time of TECELRA thaw such that it will be available for infusion when the patient is ready.
A TECELRA dose may be contained in one or more infusion bag(s). Verify the number of bags received for the indicated dose prior to preparation of TECELRA for infusion. If more than one bag will be infused for the treatment dose, thaw and administer the contents of each infusion bag completely before proceeding to thaw and infuse the contents of the next infusion bag.
1. Confirm patient identity. Prior to TECELRA preparation, match the patient's identity with the patient identifiers on each TECELRA cassette. Do not remove the TECELRA infusion bag(s) from the cassette(s) if the information on the patient-specific label does not match the patient's identity. Contact 1-855-246-9232 if there are any discrepancies between the labels and the patient identifiers.
2. Once patient identity is confirmed, remove TECELRA infusion bag(s) from the cassette(s) and check that the patient identifiers on the cassette label match the patient identifiers on the bag label. Contact 1-855-246-9232 if there are any discrepancies between the patient identifiers on the cassette and bag labels.
3. Inspect the infusion bag for any breaches of container integrity such as breaks or cracks before thawing. If the bag is compromised, do not infuse the contents and call 1-855-246-9232.
4. Place the infusion bag inside a second sealable, preferably sterile bag per institutional standard practice.
5. Thaw the infusion bag at approximately 37°C using a water bath or dry thaw method, until there is no visible ice in the infusion bag.
6. Gently mix the contents of the bag by massaging, to disperse visible cell clumps. Small clumps of cellular material should disperse with gentle manual massaging. Do not infuse TECELRA if clumps are not dispersed. Call 1-855-246-9232.
7. Keep TECELRA at ambient temperature (20°C to 25°C) once thawed. Do not pre-filter into a different container, wash, spin down, or resuspend TECELRA in new media prior to infusion.
8. Administer within one hour.
TECELRA Administration
9. Do not use a leukodepleting filter.
10. Follow universal precautions and local biosafety guidelines for handling and disposal of TECELRA to avoid potential transmission of infectious diseases, due to the presence of human blood cells that are genetically modified with replication incompetent, self-inactivating lentiviral vector.
11. Confirm patient identity with the patient identifiers on the infusion bag(s). Do not infuse TECELRA if the information on the patient-specific label does not match the intended patient. Call 1-855-246-9232. Prime the tubing of the infusion set with 0.9% sodium chloride solution prior to infusion.
12. Administer the TECELRA infusion bag via intravenous infusion within one hour. Administer the entire contents of the TECELRA infusion bag.
13. After the entire contents of the TECELRA infusion bag are infused, rinse the infusion bag with approximately 50mL 0.9% sodium chloride solution to ensure all product is delivered.
14. If more than one infusion bag has been received, administer the content of each infusion bag completely before proceeding to thaw and infuse the content of the next infusion bag, following steps 1-14 for all subsequent infusion bags.
-
3 DOSAGE FORMS AND STRENGTHS
TECELRA is a cell suspension for intravenous infusion. A single dose of TECELRA contains 2.68 x 109 to 10 x 109 MAGE-A4 TCR positive T cells in one or more infusion bag(s) [see How Supplied/Storage and Handling (16)].
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Cytokine Release Syndrome
Cytokine release syndrome (CRS), including potentially life-threatening reaction has been observed following administration of TECELRA. CRS occurred in 75% of patients, 2% of whom had Grade ≥ 3 CRS. The median time to onset was 2 days (range: 1 to 5 days) and the median time to resolution was 3 days (range: 1 to 14 days). The most common symptoms were fever (97%), tachycardia (52%), hypotension (30%), nausea/vomiting (21%) and headache (15%) [see Adverse Reactions (6)]. Management for CRS (including Grade 1) was tocilizumab (55%). Thirteen patients received one dose and five patients received more than one dose. Of the five patients who received more than one dose of tocilizumab, two patients received dexamethasone in addition to tocilizumab.
Ensure that healthcare providers administering TECELRA have immediate access to medications and resuscitative equipment to manage CRS. Ensure patients are euvolemic prior to initiating the infusions.
During and following TECELRA administration, closely monitor patients for signs and symptoms of CRS. Following treatment with TECELRA, monitor patients for at least 7 days at the healthcare facility for CRS. Continue to monitor patients for CRS for at least 4 weeks following treatment with TECELRA. Counsel patients to seek medical attention should signs or symptoms of CRS occur. At the first sign of CRS, immediately evaluate patient for hospitalization and institute treatment with supportive care based on severity and consider further management per current practice guidelines.
5.2 Immune Effector Cell-associated Neurotoxicity Syndrome
Immune Effector Cell-associated Neurotoxicity Syndrome (ICANS) has been observed following administration of TECELRA. One patient (2%) had Grade 1 ICANS. Time to onset was two days and time to resolution was one day. Symptoms included mild mental status changes. Other symptoms may include disorientation to time and place, mild drowsiness, mild inattention. Severe symptoms may include altered level of consciousness, seizures, cerebral edema, impairment of cognitive skills, progressive aphasia, motor weakness.
Ensure that healthcare providers administering TECELRA have immediate access to medications and resuscitative equipment to manage ICANS.
During and following TECELRA administration, closely monitor patients for signs and symptoms of ICANS. Following treatment with TECELRA, monitor patients for at least 7 days at the healthcare facility for ICANS. Continue to monitor patients for ICANS for at least 4 weeks following treatment with TECELRA. Counsel patients to seek medical attention should signs or symptoms of ICANS occur. At the first sign of ICANS, immediately evaluate patients for hospitalization and institute treatment with supportive care based on severity and consider further management per current practice guidelines.
Effect on Ability to Drive and Use Machines
Due to the potential for neurologic events, including dizziness and presyncope, patients receiving TECELRA are at risk for altered or decreased coordination in the 4 weeks following infusion.
Advise patients to refrain from driving and engaging in hazardous occupations or activities, such as operating heavy or potentially dangerous machinery, during this initial period.
5.3 Prolonged Severe Cytopenia
Patients may exhibit severe cytopenias, including neutropenia and thrombocytopenia [see Adverse Reactions (6)].
Patients exhibited anemia, neutropenia, and/or thrombocytopenia for several weeks following lymphodepleting chemotherapy and TECELRA infusion. Patients with Grade ≥ 3 cytopenia not resolved by week 4 included anemia (9%), neutropenia (11%), and thrombocytopenia (5%). The median time to resolution was 7.3 weeks (range: 6.1 to 8.4 weeks) for anemia, 9.3 weeks (range: 6.4 to 12.3 weeks) for neutropenia and 6.3 weeks (range: 6.1 to 6.4 weeks) for thrombocytopenia.
Monitor blood counts after TECELRA infusion. Manage cytopenia with growth factor and blood product transfusion according to local institutional guidelines/clinical practice.
5.4 Infections
Infections may occur following lymphodepleting chemotherapy and TECELRA infusion. Infections (all grades) occurred in 32% of patients with synovial sarcoma. Grade 3 or higher infections occurred in 14% of patients.
Do not administer TECELRA to patients with active infections and/or inflammatory disorders.
Monitor patients for signs and symptoms of infection before and after TECELRA infusion and treat patients appropriately.
Febrile neutropenia was observed in patients after TECELRA infusion and may be concurrent with CRS. In the event of febrile neutropenia, evaluate for infection and manage with broad-spectrum antibiotics, fluids and other supportive care, as medically indicated.
Viral reactivation has occurred in patients following treatment with TECELRA. Perform screening for Epstein-Barr Virus, Cytomegalovirus, Hepatitis B Virus, Hepatitis C Virus, Human Immunodeficiency Virus, and any other infectious agents if clinically indicated. Consider antiviral therapy to prevent viral reactivation per local guidelines.
5.5 Secondary Malignancies
Patients treated with TECELRA may develop secondary malignancies or recurrence of their cancer. Monitor for secondary malignancies.
In the event that a secondary malignancy occurs, contact 1-855-246-9232 to obtain instructions on patient samples to collect for testing.
5.6 Hypersensitivity Reactions
Serious hypersensitivity reactions, including anaphylaxis, may occur due to dimethyl sulfoxide (DMSO) in TECELRA. Observe patients for hypersensitivity reactions during infusion.
5.7 Potential for HIV Nucleic Acid Test False-Positive Results
The lentiviral vector used to make TECELRA has limited, short spans of genetic material which are identical to HIV. Therefore, some commercial HIV nucleic acid tests may yield false-positive results in patients who have received TECELRA.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety data described in this section reflects the exposure to TECELRA in 44 patients with advanced synovial sarcoma treated in the SPEARHEAD-1 clinical trial (Cohort 1). Patients with synovial sarcoma received TECELRA across a dose of 2.68 x 109 to 10 x 109 MAGE-A4 TCR positive T cells [see Clinical Studies (14)].
Serious adverse reactions occurred in 52% of patients with synovial sarcoma. The most common serious adverse reactions (occurring in ≥ 5%) included CRS (9%) and pleural effusion (7%).
Table 1 summarizes adverse reactions that occurred in at least 10% of patients.
Table 1. Adverse Reactions Occurring in ≥10% of Patients in SPEARHEAD-1 (Cohort 1) SOC
Grouped Term(N=44) All Grades
n (%)Grade ≥ 3
n (%)- * As per American Society for Transplantation and Cellular Therapy (ASTCT) criteria1
- † Any infection includes all infection terms under the 'Infections and infestations' System Organ Class
Investigations Weight decreased 5 (11) 1 (2) Gastrointestinal disorders Nausea 29 (66) 1 (2) Vomiting 16 (36) 0 (0) Constipation 14 (32) 0 (0) Abdominal pain 11 (25) 2 (5) Diarrhea 9 (21) 0 (0) General disorders and administration site conditions Fatigue 15 (34) 0 (0) Pyrexia 14 (32) 2 (5) Non-cardiac chest pain 10 (23) 1 (2) Chills 7 (16) 0 (0) Edema 9 (21) 0 (0) Asthenia 7 (16) 1 (2) Chest pain 6 (14) 0 (0) Immune system disorders Cytokine Release Syndrome* 33 (75) 1 (2) Infections and infestations Any infection† 14 (32) 6 (14) Nervous system disorders Headache 8 (18) 1 (2) Dizziness 5 (11) 0 (0) Metabolism and nutrition disorders Decreased appetite 10 (23) 1 (2) Musculoskeletal and connective tissue disorders Back pain 9 (21) 2 (5) Pain in extremity 6 (14) 0 (0) Respiratory, thoracic, and mediastinal disorders Dyspnea 11 (25) 2 (5) Cough 8 (18) 0 (0) Vascular disorders Hypotension 9 (21) 0 (0) Hypertension 7 (16) 1 (2) Cardiac disorders Sinus Tachycardia/ Tachycardia 9 (21) 0 (0) Skin and subcutaneous tissue disorders Alopecia 6 (14) 0 (0) Other clinically important adverse reactions occurring in patients receiving TECELRA include Grade 1 ICANS reported in one patient (2%).
Table 2. Laboratory Abnormalities* Worsened from Baseline in ≥10% of Patients in SPEARHEAD-1 (Cohort 1) Laboratory Abnormalities N=44 All Grades
n (%)Grade 3 or 4
n (%)Grading based on NCI CTCAE version 5.0. - * Abnormalities are laboratory values that were considered an adverse event
Lymphocyte count decreased 43 (98) 43 (98) Neutrophil count decreased 42 (96) 40 (91) White blood cell decreased 42 (96) 38 (86) Red blood cell decreased 42 (96) 14 (32) Platelet count decreased 36 (82) 9 (21) Alanine aminotransferase increased 20 (46) 2 (5) - 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data with TECELRA use in pregnant women. No animal reproductive and developmental toxicity studies have been conducted with TECELRA to assess whether it can cause fetal harm when administered to a pregnant woman. It is not known if TECELRA has the potential to be transferred to the fetus and cause fetal toxicity. Therefore, TECELRA is not recommended for women who are pregnant, and pregnancy after TECELRA administration should be discussed with the treating physician. Report all pregnancies following treatment with TECELRA to 1-855-246-9232.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the presence of TECELRA in human milk, the effect on the breastfed infant, and the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for TECELRA and any potential adverse effects on the breastfed infant from TECELRA or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Verify pregnancy status of females with reproductive potential prior to starting treatment with TECELRA.
Contraception
There are insufficient exposure data to provide a recommendation concerning duration of contraception following treatment with TECELRA.
8.4 Pediatric Use
The safety and effectiveness of TECELRA have not been established in pediatric patients.
8.5 Geriatric Use
Of the 44 patients with synovial sarcoma in the SPEARHEAD-1 study that received TECELRA, 6.8% were 65 years of age or older. Clinical studies of TECELRA did not include sufficient numbers of patients aged 65 and over to conclude whether they respond differently from younger patients.
-
11 DESCRIPTION
TECELRA (afamitresgene autoleucel) is a melanoma-associated antigen A4 (MAGE- A4)-directed genetically modified autologous T cell immunotherapy product consisting of CD4 and CD8 positive T cells transduced with a self-inactivating lentiviral vector (LV) expressing an affinity-enhanced T cell receptor (TCR) specific for the human MAGE-A4.
Autologous T cells transduced with MAGE-A4-c1032 LV express the affinity-enhanced TCR on the cell surface. The TCR recognizes an HLA-A*02 restricted MAGE-A4 peptide. MAGE-A4 is an intracellular cancer-testis antigen that has restricted expression in normal tissues and is expressed in synovial sarcoma.
TECELRA is prepared from the patient's peripheral blood mononuclear cells (PBMCs), which are obtained via a standard leukapheresis procedure. The PBMCs are enriched for T cells and are then transduced with a replication-incompetent LV containing the MAGE-A4 TCR transgene. The transduced T cells are expanded, washed, formulated into a suspension, and cryopreserved. The product must pass a sterility test before release and shipping as a frozen suspension in one or more infusion bag(s). The product is thawed prior to infusion back into the patient [see Preparation and Administration (2.2), How Supplied/Storage and Handling (16)].
The drug product formulation contains 5% dimethyl sulfoxide (DMSO).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
TECELRA is a genetically modified autologous T cell immunotherapy consisting of CD4 and CD8 positive T cells transduced with a self-inactivating LV to express an affinity- enhanced TCR specific for human MAGE-A4 on the cell surface.
The TCR recognizes an HLA-A*02 restricted MAGE-A4 peptide. MAGE-A4 is an intracellular cancer-testis antigen that has restricted expression in normal tissues and is expressed in synovial sarcoma. Antigen-specific activation of TECELRA via TCR- peptide-HLA-A*02 complex results in T cell proliferation, cytokine secretion, and killing of MAGE-A4/HLA-A*02 expressing synovial sarcoma cells.
12.2 Pharmacodynamics
In patients with synovial sarcoma who were treated with TECELRA, serum concentrations of cytokines and other soluble factors involved in cellular homeostasis, T cell activation, and inflammation (e.g. IFNγ, IL-6, IL-8, IL-15, and IL-2Rα) increased post-infusion, peaking between Days 3-8.
12.3 Pharmacokinetics
TECELRA exhibited an initial engraftment and expansion phase followed by contraction, and then persistence. High inter-individual variability was observed.
The pharmacokinetics of TECELRA in patients with synovial sarcoma are summarized in Table 3.
Table 3. Pharmacokinetics of Afamitresgene Autoleucel in SPEARHEAD-1 (Cohort 1)* PK Parameter N Statistics Value - * All patients received a dose within the range of 2.68 x 109 to 10 x 109 MAGE-A4 TCR positive T cells.
tmax (day) 44 Median (range) 7 (1-89) Cmax (DNA copies/μg) 44 Geometric mean (CV%) 189269 (109.1%) AUC0-7D (day*DNA copies/μg) 44 Geometric mean (CV%) 729653 (110.8%) AUC0-28D (day*DNA copies/μg) 41 Geometric mean (CV%) 3074205 (164.7%) AUC0-3M (day*DNA copies/μg) 35 Geometric mean (CV%) 4988965 (242.7%) AUC0-6M (day*DNA copies/μg) 33 Geometric mean (CV%) 6784047 (313.4%) Specific Populations
The pharmacokinetics of afamitresgene autoleucel (Cmax, AUC0-7D, AUC0-28D, AUC0-3M, AUC0-6M) were not impacted by body weight, body mass index, sex, age (range: 19 to 76 years), and baseline tumor sum of longest diameter (SLD).
Hepatic and renal impairment studies of TECELRA were not conducted.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity or genotoxicity studies have been conducted with TECELRA.
A genomic insertion site analysis was performed on TECELRA products from five patients. There was no evidence for preferential integration near genes of concern. No studies have been conducted to evaluate the effects of TECELRA on fertility.
-
14 CLINICAL STUDIES
Locally Inoperable/ Metastatic Synovial Sarcoma
The efficacy of TECELRA was evaluated in a multicenter, single-arm, open-label clinical trial (SPEARHEAD-1, Cohort 1). The study enrolled HLA-A*02:01P, HLA-A*02:02P, HLA-A*02:03P, and HLA-A*02:06P allele positive patients with inoperable or metastatic synovial sarcoma who had received prior systemic therapy with either doxorubicin and/or ifosfamide and whose tumor expressed the MAGE-A4 tumor antigen. The study included patients with measurable disease according to RECIST v1.1, Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and glomerular filtration rate (GFR) ≥ 60 mL/min. The study excluded patients with HLA-A*02:05P in either allele, patients on systemic corticosteroids for at least 14 days prior to leukapheresis and lymphodepletion, and recipients of allogeneic hematopoietic stem cell transplants.
Patients underwent high resolution HLA typing at a centralized testing site and had tumor samples tested for MAGE-A4 expression by an immunohistochemistry (IHC) clinical trial assay at a centralized testing site. Patients underwent leukapheresis for collection of autologous cells for processing and manufacture into TECELRA. Risk of manufacturing or delivery failure was 8% in the clinical trial (4/52) patients.
Patients received lymphodepleting chemotherapy with fludarabine 30 mg/m2/day for 4 days (Day -7 to Day -4) and cyclophosphamide 600mg/ m2/day for 3 days (Day -7 to Day -5). Patients with GFR 60-79 mL/min received an adjusted fludarabine dose of 20 mg/m2/day. TECELRA was administered as a single intravenous (IV) infusion on Day 1.
Fifty-two (52) patients were enrolled and underwent leukapheresis, eight of whom did not receive TECELRA due to the following: death (n=3), loss of eligibility prior to lymphodepleting chemotherapy (n=3), withdrawal by patient (n=1), investigator decision (n=1). Forty-five (45) patients with synovial sarcoma received lymphodepletion and one patient withdrew consent before receiving TECELRA. There were 44 patients with synovial sarcoma who received a single infusion of TECELRA.
Among the efficacy analysis population demographic characteristics were as follows: median age was 41 years (range: 19 to 73 years), 50% were female, and 89% were White, and 96% were HLA-A*02:01P.
The median number of prior lines of systemic therapies was three (range: 1 to 12 lines). Prior therapies included ifosfamide (100%), doxorubicin (95%), pazopanib (48%), trabectedin (25%), dacarbazine (11%), and gemcitabine (11%). Between leukapheresis and initiation of lymphodepletion, 16 (36%) of the 44 patients received bridging therapy. The most commonly used bridging therapy was pazopanib (69%). The median dose of TECELRA was 8x109 MAGE-A4 TCR positive T cells (range: 2.68 x 109 to 9.99 x109).
The major efficacy outcome measure was overall response rate (ORR) according to RECISTv1.1 evaluated by independent review committee (IRC). Duration of response (DOR) was an additional outcome measure.The ORR results are presented in Table 4.
Table 4. Efficacy Results* for SPEARHEAD-1 (Cohort 1) Endpoint TECELRA Treated Population
N=44CI= confidence interval; NR= not reached. - * Efficacy assessment was by independent review committee according to Response Evaluation Criteria In Solid Tumors (RECIST) v1.1.
- † Two-sided 95% confidence interval based on exact Clopper-Pearson (exact Binomial) method.
- ‡ Duration of response only applies to patients with a complete or partial response.
- § Two-sided 95% confidence interval and % of patients with response duration ≥6 and ≥12 months based on Kaplan-Meier method.
Overall Response Rate
(95% CI)†
Complete response rate, n (%)
Partial response rate, n (%)43.2%
(28.4, 59.0)
2 (4.5%)
17 (38.6%)Median Duration of Response‡ in months
(95% CI)§
Min, Max
Patients with DoR ≥ 6 months, %§
Patients with DoR ≥ 12 months, %§6.0
(4.6, NR)
1.9, 36.1+
45.6%
39.0%The median time to response from TECELRA treatment was 4.9 weeks (95% CI: 4.4 weeks, 8 weeks) by Kaplan Meier estimation.
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
TECELRA is supplied in one or more infusion bag(s) containing a frozen suspension of genetically modified autologous T cells in 5% DMSO. Each TECELRA infusion bag is individually packed in a metal cassette. Product and patient-specific labels are located on both the product infusion bag(s) and the protective shipping cassette(s).
Each infusion bag (250ml) is contained within a protective metal cassette (NDC: 83205-0001-2).
TECELRA is shipped in a liquid nitrogen dry vapor shipper at less than or equal to -130°C.
Store TECELRA in the original packaging, containing the cassette(s) protecting the infusion bag(s), in the vapor phase of liquid nitrogen at less than or equal to -130°C.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Discuss the following with the patient:
- Inform patients that there is a chance of manufacturing or delivery failure (approximately 8% in the clinical trial). Therefore, a second manufacture of TECELRA may be attempted.
- Inform patients that additional therapy (other than lymphodepletion) may be necessary before TECELRA manufacturing is completed. This may increase the risk of adverse reactions during the pre-infusion period, which could delay or prevent administration of TECELRA.
- Inform patients that following infusion, it will be necessary to be monitored daily at the healthcare facility for at least 7 days for signs and symptoms of cytokine release syndrome (CRS). Patients must remain within proximity of a healthcare facility for at least 4 weeks following infusion.
- Advise patients to seek immediate medical attention if any of the following occur:
- Cytokine Release Syndrome: inform patients that symptoms may include fever, rigors, fast heartbeat, irregular heartbeat, low blood pressure, lightheadedness or dizziness, shortness of breath, nausea/vomiting, diarrhea, and headache [see Warnings and Precautions (5.1) and Adverse Reactions (6)].
- Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS): inform patients that symptoms may include confusion, depressed level of consciousness, delirium, seizures, language difficulty [see Warnings and Precautions (5.2) and Adverse Reactions (6)].
- Bone marrow suppression and prolonged severe cytopenias: inform patients that symptoms may include bleeding or bruising, tiredness, shortness of breath, fever, pain, redness for several weeks following lymphodepleting chemotherapy and TECELRA blood counts before and after TECELRA infusion should be periodically monitored [see Warnings and Precautions (5.3) and Adverse Reactions (6)].
- Infections: inform patients that they may exhibit signs or symptoms associated with infection, and that past infections can be reactivated following treatment with TECELRA [see Warnings and Precautions (5.4) and Adverse Reactions (6)].
Advise patients for the need to:
- Contact 1-855-246-9232 if they are diagnosed with a secondary malignancy [see Warnings and Precautions (5.5)].
- Refrain from driving or operating heavy or potentially dangerous machines for at least 4 weeks after TECELRA administration [see Warnings and Precautions (5.2)].
Manufactured by: USWM CT, LLC
351 Rouse Boulevard
Philadelphia, PA 19112
U.S. License Number 2416© 2026 USWM CT, LLC.
-
MEDICATION GUIDE
Medication Guide
TECELRA® (pronounced tuh-sel-ruh)
(afamitresgene autoleucel)Issued: Aug 2024 Read this Medication Guide before you start your TECELRA treatment. Talk with your healthcare provider if you have questions about your health condition or treatment. Reading this Medication Guide does not take the place of talking with your healthcare provider about your treatment. What is the most important information I should know about TECELRA?
You will likely be in a hospital before and after getting TECELRA.
TECELRA may cause side effects that can be severe or life-threatening. Call your healthcare provider or get emergency help right away if you get any of the following:
Fever (100.4°F/38°C or higher)
Chills/Shivering
Difficulty breathing
Fast or irregular heartbeat
Low blood pressure
Fatigue
Severe nausea, vomiting, or diarrhea
Severe headache
New skin rash
Tell all your healthcare providers that you were treated with TECELRA.What is TECELRA?
TECELRA is a medicine, called a genetically modified autologous T cell immunotherapy, that is used to treat synovial sarcoma. It is used when other kinds of treatment do not work.
TECELRA is different from other cancer medicines because it is made from your own white blood cells that are made to recognize and attack your cancer cells.
Your healthcare provider will perform tests to see if TECELRA is right for you.
Before you get TECELRA, tell your healthcare provider about all your medical problems, including:
Seizure, stroke, confusion, or memory loss
Heart, liver or kidney problems
Low blood pressure
Lung or breathing problems
Recent or active infection
Past infections which can be reactivated following treatment with TECELRA
Low blood counts
Pregnancy, you think you may be pregnant, or plan to become pregnant
Breastfeeding
Taking a blood thinner
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How will I get TECELRA?
Since TECELRA is made from your own white blood cells, your blood will be collected by a process called "leukapheresis" (loo-kah-fur-ee-sis). This gets sent to a company to make the TECELRA for you.
It takes about 6 weeks to get your TECELRA back, but the time may vary.
While your TECELRA is being made, your healthcare provider may give you other medicines to stabilize your cancer.
Before you get your TECELRA, you will get 4 days of chemotherapy to prepare your body.
When your TECELRA is ready, you get a tube (intravenous catheter) placed into your vein and your dose of TECELRA will be given in one or more infusion bags. The infusion may take up to 60 minutes for each infusion bag.
After getting TECELRA, you will be monitored daily at the healthcare facility, for at least 7 days after the infusion.
You should plan to stay close to a healthcare facility for at least 4 weeks. Your healthcare provider will check to see that your treatment is working and help you with any side effects that may occur.
Your healthcare provider will do blood tests to follow your progress. It is important that you have your blood tested. If you miss a scheduled appointment for your collection of blood, call your healthcare provider as soon as possible to reschedule.What are the possible or reasonably likely side effects of TECELRA?
The most common side effects of TECELRA include:
Nausea
Vomiting
Fatigue
Constipation
Fever (100.4°F/38°C or higher)
Infection
Abdominal pain
Difficulty breathing
Decreased appetite
Diarrhea
Low blood pressure
Back pain
Fast heart rate
Chest pain
General body swelling
Low white blood cells
Low red blood cells
Low platelets
What should I avoid after receiving TECELRA?
Do not drive, operate heavy machinery, or do other activities that could be dangerous for at least 4 weeks after you get TECELRA.
Do not donate blood, organs, tissues, or cells for transplantation.
What are the ingredients in TECELRA?
Active ingredient: afamitresgene autoleucel
Inactive ingredients: Dimethyl Sulfoxide (DMSO)
-
PRINCIPAL DISPLAY PANEL - Bag and Cassette Label
afamitresgene autoleucel
Tecelra®NDC: 83205-0001-2
Rx ONLY
FOR AUTOLOGOUS & INTRAVENOUS USE ONLY
No U.S. standard of potencyContains: 2.68 x 109 to 10 x 109 MAGE-A4 TCR positive T cells in
frozen suspension containing 5% DMSO (no preservative)Gently mix by massaging
post-thaw.
See full prescribing information for
instructions for administration.
Store and transport at ≤ -130°C.Suspension for intravenous infusion
GENETICALLY MODIFIED
DO NOT USE A LEUKODEPLETING
FILTER OR IRRADIATE
Not evaluated for infectious substancesManufacturer: USWM CT, LLC, Philadelphia, PA 19112 USA
Phone: 1-855-246-9232 U.S. Lic. #2416LBL 00034 Rev 02

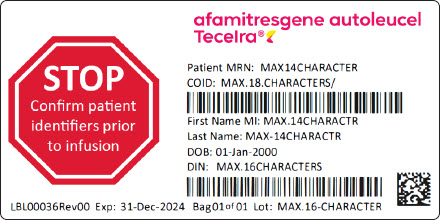
- PRINCIPAL DISPLAY PANEL - Bag Label - Patient Identifier
- PRINCIPAL DISPLAY PANEL - Cassette Label - Patient Identifier
-
INGREDIENTS AND APPEARANCE
TECELRA
afamitresgene autoleucel injection, suspensionProduct Information Product Type CELLULAR THERAPY Item Code (Source) NDC: 83205-0001 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AFAMITRESGENE AUTOLEUCEL (UNII: CUY18BJ7BP) (AFAMITRESGENE AUTOLEUCEL - UNII:CUY18BJ7BP) AFAMITRESGENE AUTOLEUCEL 10000000000 Inactive Ingredients Ingredient Name Strength DIMETHYL SULFOXIDE (UNII: YOW8V9698H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 83205-0001-2 1 in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125789 08/02/2024 Labeler - USWM, LLC (117542566) Registrant - Adaptimmune LLC (078438854) Establishment Name Address ID/FEI Business Operations USWM CT, LLC 144859435 ANALYSIS(83205-0001) , MANUFACTURE(83205-0001) , API MANUFACTURE(83205-0001) , PACK(83205-0001) , LABEL(83205-0001)
Trademark Results [TECELRA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TECELRA 98241205 not registered Live/Pending |
Adaptimmune Limited 2023-10-26 |
 TECELRA 90452726 not registered Live/Pending |
Adaptimmune Limited 2021-01-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.