Antiseptic Wipes by Kikkerland Design Inc. / Ni Hau Industrial Co., Ltd. Kikkerland 009-01
Antiseptic Wipes by
Drug Labeling and Warnings
Antiseptic Wipes by is a Otc medication manufactured, distributed, or labeled by Kikkerland Design Inc., Ni Hau Industrial Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
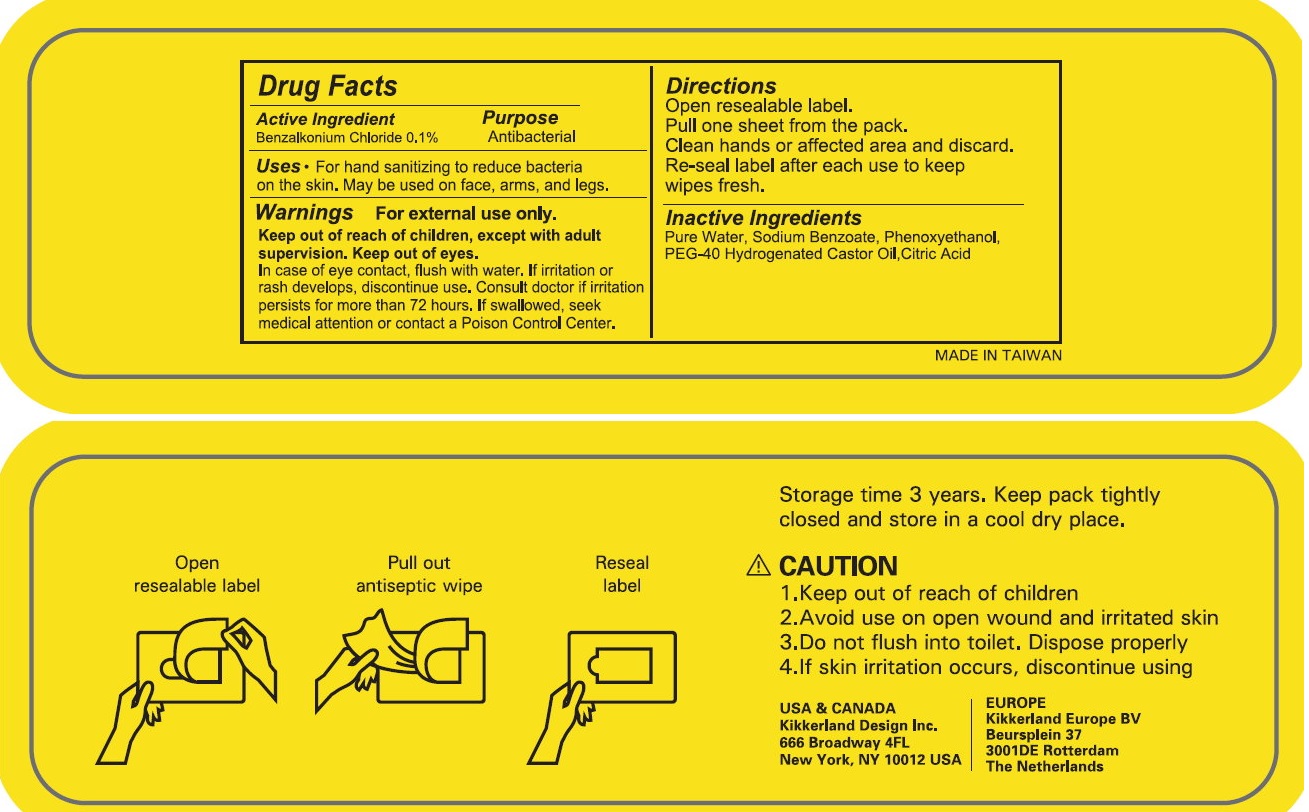
ANTISEPTIC WIPES- benzakonium chloride cloth
Kikkerland Design Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Kikkerland 009-01
Keep out of reach of children, except with adult supervision. If swallowed, seek medical attention or contact a Poison Control Center
Directions
Open resealable label.
Pull one sheet from the pack.
Clean hands or affected area and discard.
Re-seal label after each use to keep wipes fresh.
| ANTISEPTIC WIPES
benzakonium chloride cloth |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Kikkerland Design Inc. (858591548) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ni Hau Industrial Co., Ltd. | 658274464 | manufacture(79138-009) | |
Revised: 7/2023
Document Id: 002bc576-0ef4-96d7-e063-6294a90ae5de
Set id: ab24e063-0126-092c-e053-2995a90afc66
Version: 5
Effective Time: 20230710
 10 Wipes NDC:
10 Wipes NDC: