HAND SANITIZER by JUXIN (Jiangsu) New Material Packaging Co., Ltd. HAND SANITIZER gel
HAND SANITIZER by
Drug Labeling and Warnings
HAND SANITIZER by is a Otc medication manufactured, distributed, or labeled by JUXIN (Jiangsu) New Material Packaging Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
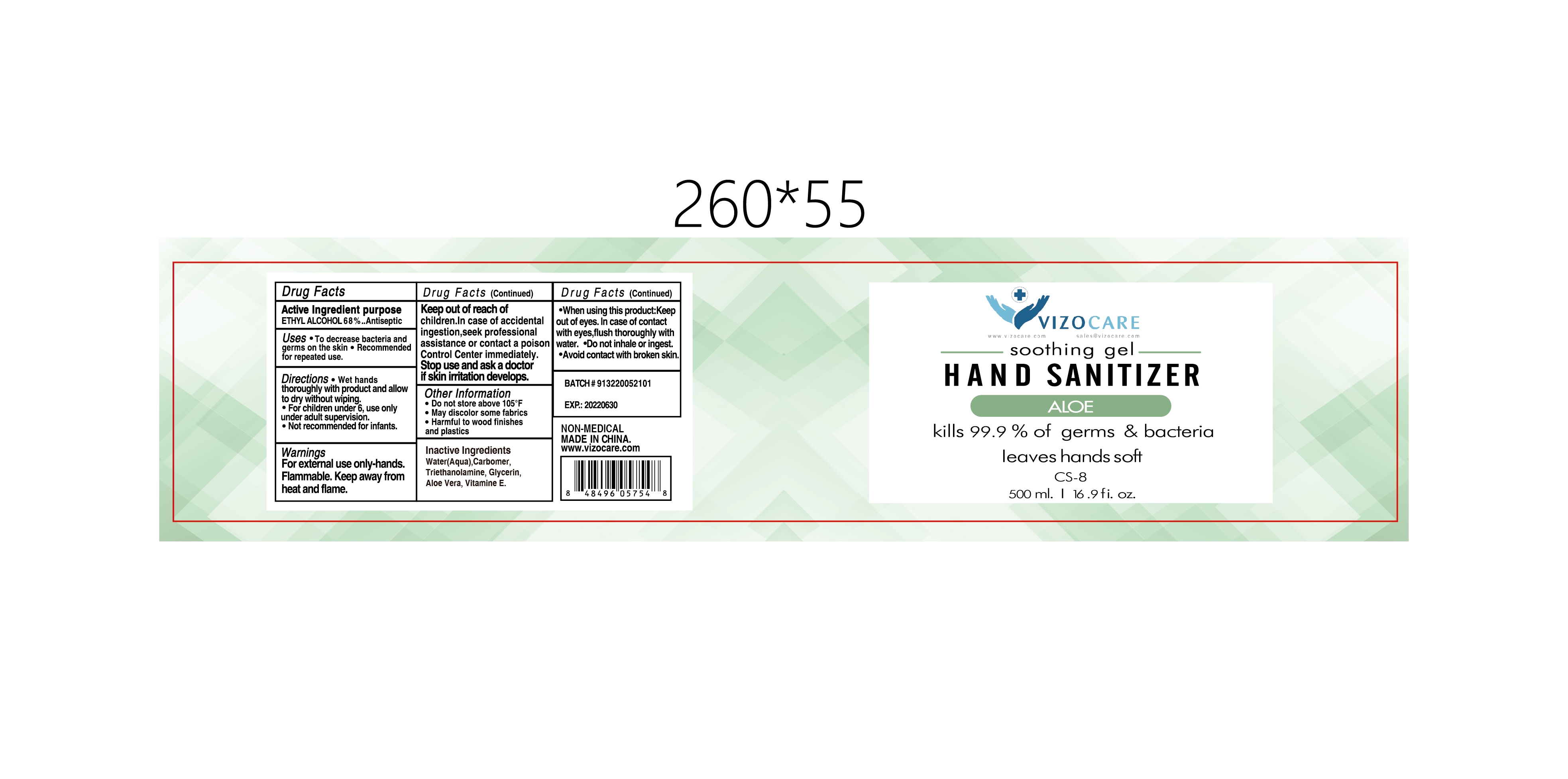
- Active Ingredient(s)
- Purpose
- Use
- Warnings
- Do not use
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive ingredients
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
HAND SANITIZER
hand sanitizer gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 79805-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 68 mL in 100 mL Inactive Ingredients Ingredient Name Strength TROLAMINE (UNII: 9O3K93S3TK) ALOE VERA LEAF (UNII: ZY81Z83H0X) GLYCERIN (UNII: PDC6A3C0OX) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) WATER (UNII: 059QF0KO0R) .ALPHA.-TOCOPHEROL SUCCINATE, D- (UNII: LU4B53JYVE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 79805-001-01 60 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/30/2020 2 NDC: 79805-001-02 120 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/30/2020 3 NDC: 79805-001-03 300 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/30/2020 4 NDC: 79805-001-04 500 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/30/2020 5 NDC: 79805-001-05 3800 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/30/2020 6 NDC: 79805-001-06 90 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/30/2020 7 NDC: 79805-001-07 100 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/30/2020 8 NDC: 79805-001-08 750 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/30/2020 9 NDC: 79805-001-09 1000 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/30/2020 10 NDC: 79805-001-12 2000 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/30/2020 11 NDC: 79805-001-11 250 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/30/2020 12 NDC: 79805-001-10 18 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/30/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/30/2020 Labeler - JUXIN (Jiangsu) New Material Packaging Co., Ltd. (546994282) Establishment Name Address ID/FEI Business Operations JUXIN (Jiangsu) New Material Packaging Co., Ltd. 546994282 manufacture(79805-001)
Trademark Results [HAND SANITIZER]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 HAND SANITIZER 88958909 not registered Live/Pending |
MAISON BLANCHE, LLC 2020-06-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.