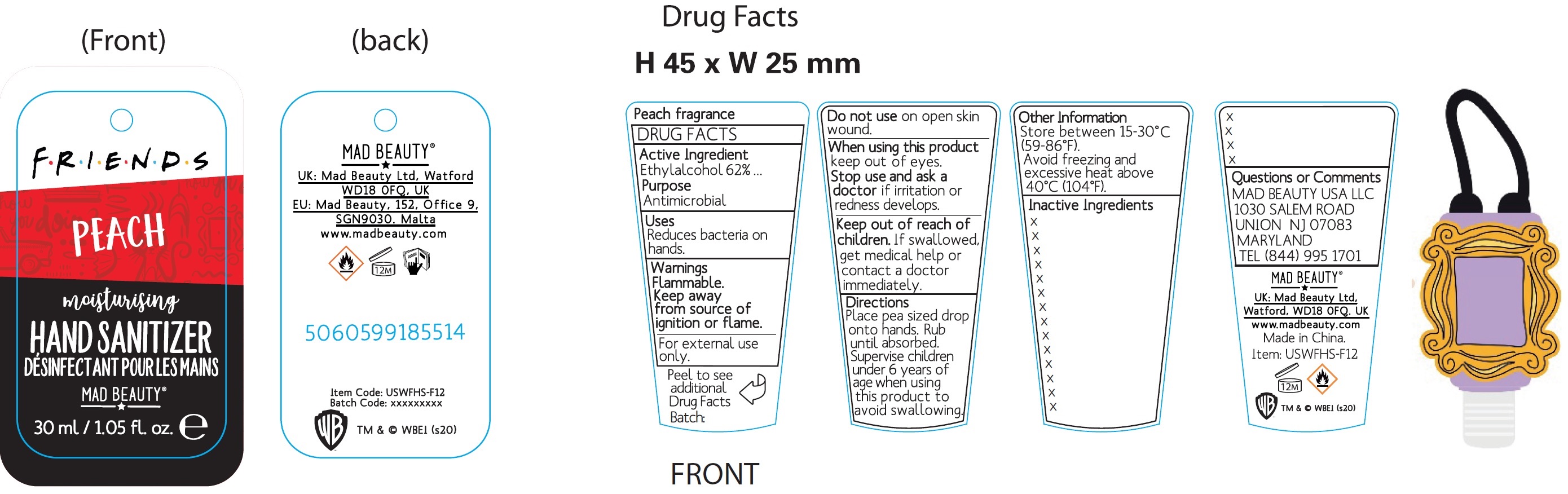
Warner Friends Moisturizing Hand Sanitizer, Peach
Warner Friends Moisturizing Hand Sanitizer Peach by
Drug Labeling and Warnings
Warner Friends Moisturizing Hand Sanitizer Peach by is a Otc medication manufactured, distributed, or labeled by Mad Beauty USA LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
WARNER FRIENDS MOISTURIZING HAND SANITIZER PEACH- alcohol gel
Mad Beauty USA LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Warner Friends Moisturizing Hand Sanitizer, Peach
Directions
Place pea sized drop onto hands. Rub until absorbed. Supervise children under 6 years of age when using this product to avoid swallowing.
| WARNER FRIENDS MOISTURIZING HAND SANITIZER PEACH
alcohol gel |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Mad Beauty USA LLC (117508758) |
Revised: 12/2021
Document Id: d2bd01b1-7065-3c3f-e053-2995a90a562c
Set id: ab32fbc1-ec6a-ba7c-e053-2a95a90a0155
Version: 2
Effective Time: 20211209
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.