FEXOFENADINE HYDROCHLORIDE tablet, film coated
Fexofenadine hydrochloride by
Drug Labeling and Warnings
Fexofenadine hydrochloride by is a Otc medication manufactured, distributed, or labeled by Sunmark, Aurolife Pharma, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient(in each tablet)
- Purpose
- Uses
- Warnings
- Do not use
- Ask a doctor before use if you have
- When using this product
- Stop use and ask doctor if
- If pregnant or breast-feeding
- Keep out of reach of children
-
Directions
adults and children 12 years of age and over take one 180 mg tablet with water once a day;
do not take more than 1 tablet in 24 hourschildren under 12 years of age do not use adults 65 years of age and older ask a doctor consumers with kidney disease ask a doctor
adults and children 12 years of age and over take one 60 mg tablet with water every 12 hours;
do not take more than 2 tablet in 24 hourschildren under 12 years of age do not use adults 65 years of age and older ask a doctor consumers with kidney disease ask a doctor - Other information
- Inactive ingredients
- Questions or comments?
-
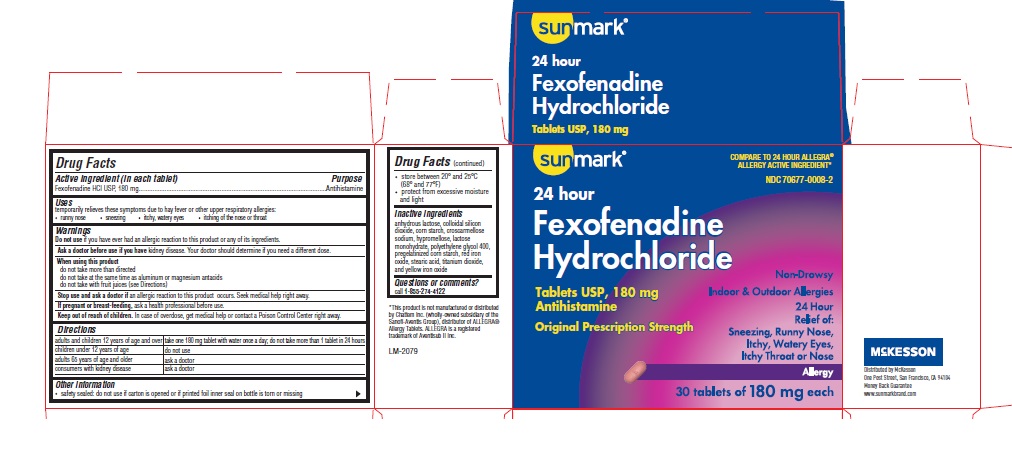
Principal Display Panel
NDC: 70677-0008-2
Original Prescription Strength
Non-Drowsy
Fexofenadine Hydrochloride Tablets USP, 180 mg/antihistamine
Allergy
Indoor & Outdoor Allergies
24 Hours Relief of:
Sneezing
Runny nose
Itchy, Watery Eyes
Itchy Throat or Nose
DO NOT USE IF FOIL SEAL IS TORN OR MISSING
30 Tablets 180 mg each
-
Principal Display Panel
NDC: 70677-0007-1
Original Prescription Strength
Non-Drowsy
Fexofenadine Hydrochloride Tablets USP, 60 mg/antihistamine
Allergy
Indoor & Outdoor Allergies
12 Hours Relief of:
Sneezing
Runny nose
Itchy, Watery Eyes
Itchy Throat or Nose
DO NOT USE IF FOIL SEAL IS TORN OR MISSING
12 Tablets 60 mg each

-
INGREDIENTS AND APPEARANCE
FEXOFENADINE HYDROCHLORIDE
fexofenadine hydrochloride tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 70677-0008 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 180 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSES (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STARCH, CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color ORANGE (Peach) Score no score Shape CAPSULE (Bevel Edge, Biconvex) Size 17mm Flavor Imprint Code E;44 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70677-0008-2 30 in 1 BOTTLE; Type 0: Not a Combination Product 10/12/2016 2 NDC: 70677-0008-1 15 in 1 BLISTER PACK; Type 0: Not a Combination Product 10/12/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202039 10/12/2016 FEXOFENADINE HYDROCHLORIDE
fexofenadine hydrochloride tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 70677-0007 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 60 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSES (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STARCH, CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color ORANGE (peach) Score no score Shape CAPSULE (Bevel Edge, Biconvex) Size 12mm Flavor Imprint Code E;42 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70677-0007-1 12 in 1 BLISTER PACK; Type 0: Not a Combination Product 10/12/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202039 10/12/2016 Labeler - Sunmark (116956644) Registrant - Aurolife Pharma, LLC (829084461) Establishment Name Address ID/FEI Business Operations Aurolife Pharma, LLC 829084461 MANUFACTURE(70677-0007, 70677-0008)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.