L-Topical POVIDONE IODINE, 7.5% SCRUB
L-Topical POVIDONE IODINE, 7.5% SCRUB by
Drug Labeling and Warnings
L-Topical POVIDONE IODINE, 7.5% SCRUB by is a Otc medication manufactured, distributed, or labeled by GLOBAL PHARMA HEALTHCARE PRIVATE LIMITED. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
L-TOPICAL POVIDONE IODINE, 7.5% SCRUB- povidone iodine liquid
GLOBAL PHARMA HEALTHCARE PRIVATE LIMITED
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
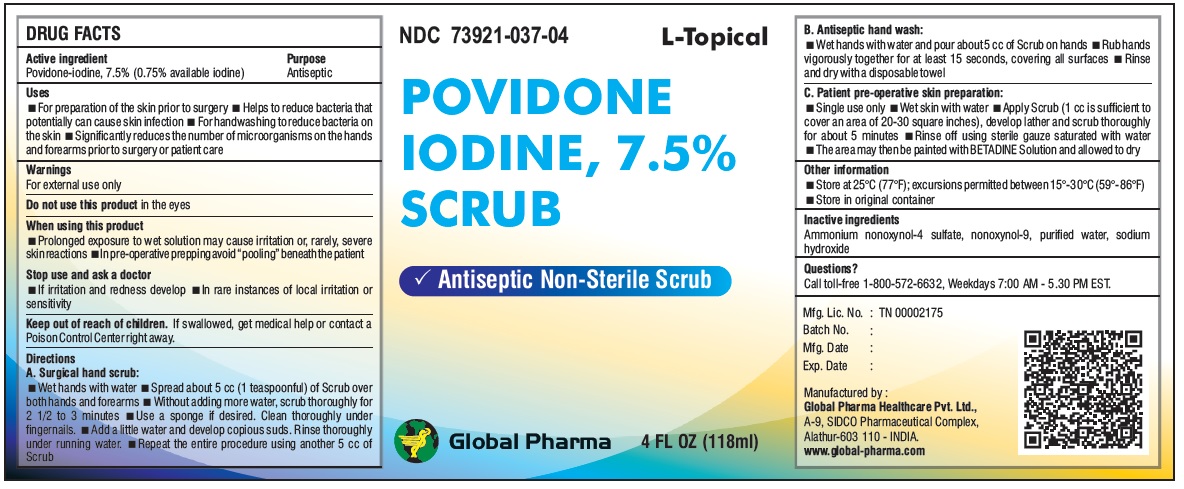
L-Topical POVIDONE IODINE, 7.5% SCRUB
Uses
For preparation of the skin prior to surgery Helps to reduce bacteria that potentially can cause skin infection For handwashing to reduce bacteria on the skin Significantly reduces the number of microorganisms on the hands and forearms prior to surgery or patient care
Warnings
For external use only
Do not use this product in the eyes
When using this product
Prolonged exposure to wet solution may cause irritation or, rarely, severe skin reactions In pre-operative prepping avoid “pooling” beneath the patient
Stop use and ask a doctor
If irritation and redness develop In rare instances of local irritation or sensitivity
Directions
A. Surgical hand scrub:
Wet hands with water Spread about 5 cc (1 teaspoonful) of Scrub over both hands and forearms Without adding more water, scrub thoroughly for 2 1/2 to 3 minutes Use a sponge if desired. Clean thoroughly under fingernails. Add a little water and develop copious suds. Rinse thoroughly under running water. Repeat the entire procedure using another 5 cc of Scrub
B. Antiseptic hand wash:
Wet hands with water and pour about 5 cc of Scrub on hands Rub hands vigorously together for at least 15 seconds, covering all surfaces Rinse and dry with a disposable towel
C. Patient pre-operative skin preparation:
Single use only Wet skin with water Apply Scrub (1 cc is sufficient to cover an area of 20-30 square inches), develop lather and scrub thoroughly for about 5 minutes Rinse off using sterile gauze saturated with water The area may then be painted with BETADINE Solution and allowed to dry
Other information
Store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
Store in original container
| L-TOPICAL POVIDONE IODINE, 7.5% SCRUB
povidone iodine liquid |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - GLOBAL PHARMA HEALTHCARE PRIVATE LIMITED (860186917) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| GLOBAL PHARMA HEALTHCARE PRIVATE LIMITED | 860186917 | manufacture(73921-037) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.