Qutenza by Acorda Therapeutics, Inc. / Sharp Corporation / Contract Pharmaceuticals Limited Canada - Lab Svcs (CPL) / SGS Life Science Services / Exova Canada Inc. / Contract Pharmaceuticals Limited Canada (CPL) / SGS Institut Fresenius GmbH / Lohmann Therapie-Systeme AG (LTS) (Germany) QUTENZA- capsaicin kit
Qutenza by
Drug Labeling and Warnings
Qutenza by is a Prescription medication manufactured, distributed, or labeled by Acorda Therapeutics, Inc., Sharp Corporation, Contract Pharmaceuticals Limited Canada - Lab Svcs (CPL), SGS Life Science Services, Exova Canada Inc., Contract Pharmaceuticals Limited Canada (CPL), SGS Institut Fresenius GmbH, Lohmann Therapie-Systeme AG (LTS) (Germany). Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use QUTENZA safely and effectively. See full prescribing information for QUTENZA.
QUTENZA ® (capsaicin) 8% patch
Initial U.S. Approval: 2009INDICATIONS AND USAGE
- Qutenza is a TRPV1 channel agonist indicated for the management of neuropathic pain associated with postherpetic neuralgia (PHN). ( 1)
DOSAGE AND ADMINISTRATION
- Only physicians or health care professionals under the close supervision of a physician are to administer Qutenza. ( 2.1)
- Do not use Qutenza on broken skin. ( 2.1)
- Apply Qutenza to the most painful skin areas, using up to four patches. ( 2.2)
- Apply Qutenza for 60 minutes and repeat every 3 months or as warranted by the return of pain (not more frequently than every three months). ( 2.2)
- Use only nitrile (not latex) gloves when handling Qutenza and when cleaning treatment areas. ( 2.1)
- Before patch application, a physician must identify and mark the painful area, including areas of hypersensitivity and allodynia. ( 2.3)
- Apply a topical anesthetic before Qutenza application. ( 2.3)
- Apply Qutenza by placing on the skin while slowly removing the release liner from underneath. ( 2.3)
- Remove the Qutenza patches by gently and slowly rolling them inward. ( 2.3)
- After removal of Qutenza, apply Cleansing Gel for one minute and then remove it with a dry wipe. ( 2.3)
- Treat acute pain during and following the procedure with local cooling and/or analgesics. ( 5.4)
- Dispose of patches and other treatment materials immediately after use in accordance with local biomedical waste procedures. ( 2.1)
- The treated area may be sensitive for a few days to heat (e.g., hot showers/baths, direct sunlight, vigorous exercise). ( 2.3)
DOSAGE FORMS AND STRENGTHS
- Qutenza patch contains 8% capsaicin (640 mcg/cm 2). Each patch contains a total of 179 mg of capsaicin. ( 3)
CONTRAINDICATIONS
- None
WARNINGS AND PRECAUTIONS
- Do not use near eyes or mucous membranes. ( 5.1)
- Inhalation of airborne capsaicin can result in coughing or sneezing. ( 5.2)
- If irritation of eyes or airway occurs, remove the affected individual from the vicinity of Qutenza and flush the mucous membranes or eyes with water. If skin not intended to be treated comes into contact with Qutenza, apply Cleansing Gel and then wipe off with dry gauze. ( 5.2, 5.3)
- Transient increases in blood pressure may occur in patients during and shortly after the Qutenza treatment. Monitor blood pressure during and following the treatment procedure. For those patients who require the use of opioids to treat pain during or following the procedure, their ability to perform potentially hazardous activities such as driving or operating machinery may be affected. ( 5.4, 5.5)
ADVERSE REACTIONS
The most common adverse reactions (≥ 5% and greater than control) are application site erythema, application site pain, application site pruritus and application site papules. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Acorda Therapeutics at 1-877-900-6479 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2013
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Special Precautions
2.2 Dosing
2.3 Instructions for Use
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Eye and Mucous Membrane Exposure
5.2 Aerosolization of Capsaicin
5.3 Unintended Skin Exposure
5.4 Application Associated Pain
5.5 Increase in Blood Pressure
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Postherpetic Neuralgia
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage
16.3 Handling and Disposal
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Special Precautions
- Only physicians or health care professionals under the close supervision of a physician are to administer Qutenza.
- Use only nitrile gloves when handling Qutenza, and when cleaning capsaicin residue from the skin. Do not use latex gloves as they do not provide adequate protection.
- Immediately after use, dispose of used and unused patches, Cleansing Gel and other treatment materials in accordance with the local biomedical waste procedures.
- Use Qutenza only on dry, intact (unbroken) skin.
- Apply the Qutenza patch within 2 hours of opening the pouch.
2.2 Dosing
The recommended dose of Qutenza is a single, 60-minute application of up to four patches.
Treatment with Qutenza may be repeated every three months or as warranted by the return of pain (not more frequently than every three months).
2.3 Instructions for Use
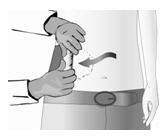
Identify
The treatment area (painful area including areas of hypersensitivity and allodynia) must be identified by a physician and marked on the skin.
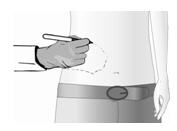
If necessary, clip hair (do not shave) in and around the identified treatment area to promote patch adherence.
Qutenza can be cut to match the size and the shape of the treatment area.
Gently wash the treatment area with mild soap and water and dry thoroughly.
Anesthetize
Pre-treat with a topical anesthetic to reduce discomfort associated with the application of Qutenza.
Apply topical anesthetic to the entire treatment area and surrounding 1 to 2 cm, and keep the local anesthetic in place until the skin is anesthetized prior to the application of Qutenza patch.
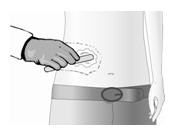
Remove the topical anesthetic with a dry wipe. Gently wash the treatment area with mild soap and water and dry thoroughly.
Apply
Tear open the pouch along the three dashed lines, remove the Qutenza patch.
Inspect the Qutenza patch and identify the outer surface backing layer with the printing on one side and the capsaicin-containing adhesive on the other side. The adhesive side of the patch is covered by a clear, unprinted, diagonally-cut release liner.
Cut Qutenza before removing the protective release liner.
The diagonal cut in the release liner is to aid in its removal. Peel a small section of the release liner back, and place the adhesive side of the patch on the treatment area.
While you slowly peel back the release liner from under the patch with one hand, use your other hand to smooth the patch down on to the skin.
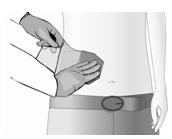
Once Qutenza is applied, leave in place for 60 minutes.
To ensure Qutenza maintains contact with the treatment area, a dressing, such as rolled gauze, may be used.
Instruct the patient not to touch the patch or treatment area.
Cleanse
After removal of Qutenza, generously apply Cleansing Gel to the treatment area and leave on for at least one minute. Remove Cleansing Gel with a dry wipe and gently wash the area with mild soap and water and dry thoroughly.
Dispose of all treatment materials as described. [ see Dosage and Administration (2.1) ]
Inform the patient that the treated area may be sensitive for a few days to heat (e.g., hot showers/baths, direct sunlight, vigorous exercise).
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Eye and Mucous Membrane Exposure
Do not apply Qutenza to the face or scalp to avoid risk of exposure to the eyes or mucous membranes.
5.2 Aerosolization of Capsaicin
Aerosolization of capsaicin can occur upon rapid removal of Qutenza patches. Therefore, remove Qutenza patches gently and slowly by rolling the adhesive side inward [see Dosage and Administration (2.3)] .
If irritation of eyes or airways occurs, remove the affected individual from the vicinity of Qutenza. Flush eyes and mucous membranes with cool water.
Inhalation of airborne capsaicin can result in coughing or sneezing. Provide supportive medical care if shortness of breath develops.
5.3 Unintended Skin Exposure
If skin not intended to be treated comes in contact with Qutenza, apply Cleansing Gel for one minute and wipe off with dry gauze. After the Cleansing Gel has been wiped off, wash the area with soap and water.
5.4 Application Associated Pain
Even following use of a local anesthetic prior to administration of Qutenza, patients may experience substantial procedural pain. Prepare to treat acute pain during and following the application procedure with local cooling (such as an ice pack) and/or appropriate analgesic medication, such as opioids. Opioids may affect the ability to perform potentially hazardous activities such as driving or operating machinery.
5.5 Increase in Blood Pressure
In clinical trials, increases in blood pressure occurred during or shortly after exposure to Qutenza. The changes averaged less than 10 mm Hg, although some patients had greater increases and these changes lasted for approximately two hours after patch removal. Increases in blood pressure were unrelated to the pretreatment blood pressure but were related to treatment-related increases in pain. Monitor blood pressure periodically during the treatment and provide adequate support for treatment related pain.
Patients with unstable or poorly controlled hypertension, a recent history of cardiovascular or cerebrovascular events may be at an increased risk of adverse cardiovascular effects. Consider these factors prior to initiating Qutenza treatment.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed elsewhere in the labeling:
Application-Associated Pain [ see Warnings and Precautions (5.4)]
Increase in Blood Pressure [ see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of other drugs and may not reflect the rates observed in clinical practice.
Across all controlled and uncontrolled trials, more than 1,600 patients have received Qutenza. A total of 394 patients received more than one treatment application and 274 patients were followed for 48 weeks or longer.
In controlled clinical studies, 98% of patients completed ≥ 90% of the intended patch application duration. Among patients treated with Qutenza, 1% discontinued prematurely due to an adverse event.
Controlled Clinical Studies
Common Adverse Reactions
Adverse reactions occurring in ≥ 5% of patients in the Qutenza group and at an incidence greater than in the control group were application site erythema, application site pain, application site pruritus and application site papules.
Table 1 summarizes all adverse reactions, regardless of causality, occurring in ≥ 1% of patients with postherpetic neuralgia in the Qutenza group for which the incidence was greater than in the control group. The majority of application site reactions were transient and self-limited. Transient increases in pain were commonly observed on the day of treatment in patients treated with Qutenza. Pain increases occurring during patch application usually began to resolve after patch removal. On average, pain scores returned to baseline by the end of the treatment day and then remained at or below baseline levels. A majority of Qutenza-treated patients in clinical studies had adverse reactions with a maximum intensity of "mild" or "moderate".
TABLE 1: Treatment-emergent adverse reaction incidence (%) in controlled trials in Postherpetic Neuralgia (Events in ≥ 1% of Qutenza-treated patients and at least 1% greater in the Qutenza group than in the Control group) Body System
Preferred TermQutenza
60 minutes
(N = 622)
%Control
60 minutes
(N = 495)
%General Disorders and Administration Site Conditions Application Site Erythema 63 54 Application Site Pain 42 21 Application Site Pruritus 6 4 Application Site Papules 6 3 Application Site Edema 4 1 Application Site Swelling 2 1 Application Site Dryness 2 1 Infections and Infestations Nasopharyngitis 4 2 Bronchitis 2 1 Sinusitis 3 1 Gastrointestinal Disorders Nausea 5 2 Vomiting 3 1 Skin and Subcutaneous Tissue Disorder Pruritus 2 < 1 Vascular Disorders Hypertension 2 1 Other Adverse Reactions Observed During the Clinical Studies of Qutenza
General Disorders and Administration Site Conditions: Application site urticaria, Application site paresthesia, Application site dermatitis, Application site hyperesthesia, Application site excoriation, Application site warmth, Application site anesthesia, Application site bruising, Application site inflammation, Application site exfoliation, Peripheral edema
Nervous System Disorders: Headache, Burning sensation, Peripheral sensory neuropathy, Dizziness, Dysgeusia, Hyperesthesia, Hypoesthesia
Respiratory, Thoracic and Mediastinal Disorders: Cough, Throat irritation
Skin and Subcutaneous Tissue Disorders: Abnormal skin odor
-
7 DRUG INTERACTIONS
No clinical drug interaction studies have been performed.
Data from in vitro cytochrome P450 inhibition and induction studies show that capsaicin does not inhibit or induce liver cytochrome P450 enzymes at concentrations which far exceed those measured in blood samples. Therefore, interactions with systemic medicinal products are unlikely.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic effects: Pregnancy Category B
There are no adequate and well-controlled studies evaluating Qutenza in pregnant women.
There was no evidence of fetal teratogenicity in embryofetal developmental toxicological studies conducted in pregnant rats and rabbits in which Qutenza patches (rats) or liquid (rabbits) were applied once daily for a 3 hour duration during the period of fetal organogenesis up to doses corresponding to an 11-fold (rat, 32 mg capsaicin patch/day) and 37-fold (rabbit, 260 mg capsaicin/day) margin over the maximum recommended human dose [MRHD] based on a C max exposure comparison.
In a peri- and post-natal reproduction toxicology study, pregnant female rats were treated with Qutenza patches at doses up to 32 mg capsaicin patch/rat/day applied once daily for a 3 hours duration during gestation and lactation (from gestation day 7 through day 28 postpartum). Analyses of milk samples on day 14 of the lactation period demonstrated measurable levels of capsaicin in the dam's milk at all dose levels. There were no effects on survival, growth, learning and memory tests (passive avoidance and water maze), sexual maturation, mating, pregnancy, and fetal development in the offspring of mothers treated with capsaicin up to 32 mg capsaicin patch/rat/day (corresponding to an 11-fold margin over the MRHD based on C max exposure).
8.3 Nursing Mothers
There are no adequate and well-controlled studies in nursing women. Studies in rat have demonstrated capsaicin is excreted into breast milk of this species. It is unknown whether capsaicin is excreted in human breast milk. Because Qutenza is administered as a single 60-minute application and capsaicin is rapidly cleared from the bloodstream [ see Clinical Pharmacology (12.3) ], mothers can reduce infant exposure by not breast-feeding after treatment on the day of treatment.
8.4 Pediatric Use
The safety and effectiveness of Qutenza in patients younger than 18 years of age have not been studied.
8.5 Geriatric Use
In controlled clinical studies of Qutenza in neuropathic pain associated with postherpetic neuralgia, 75% of patients were 65 years and older and 43% of patients were 75 years and older.
Safety and effectiveness were similar in geriatric patients and younger patients. No dose adjustments are required in geriatric patients.
-
10 OVERDOSAGE
There is no clinical experience with Qutenza overdose in humans.
There is no specific antidote for overdose with capsaicin. In case of suspected overdose, remove patches gently, apply Cleansing Gel for one minute, wipe off with dry gauze and gently wash the area with soap and water. Use supportive measures and treat symptoms as clinically warranted.
-
11 DESCRIPTION
Qutenza (capsaicin) 8% patch contains capsaicin in a localized dermal delivery system. The capsaicin in Qutenza is a synthetic equivalent of the naturally occurring compound found in chili peppers. Capsaicin is soluble in alcohol, acetone, and ethyl acetate and very slightly soluble in water.
Qutenza is a single-use patch stored in a foil pouch. Each Qutenza patch is 14 cm x 20 cm (280 cm 2) and consists of a polyester backing film coated with a drug-containing silicone adhesive mixture, and covered with a removable polyester release liner.
The backing film is imprinted with "capsaicin 8%". Each Qutenza patch contains a total of 179 mg of capsaicin (8% in adhesive, 80 mg per gram of adhesive) or 640 micrograms (mcg) of capsaicin per square cm of patch.
The empirical formula is C 18H 27NO 3, with a molecular weight of 305.42. The chemical compound capsaicin [(E)-8-methyl-N-vanillyl-6-nonenamide] is an activating ligand for transient receptor potential vanilloid 1 receptor (TRPV1) and it has the following structure:
FIGURE 1:
Structural Formula of Capsaicin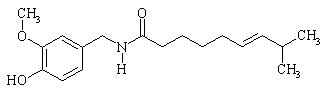
The patch contains the following inactive ingredients: diethylene glycol monoethyl ether, dimethicone, ethyl cellulose, polyester film, silicone adhesive and white ink.
Qutenza is supplied with a Cleansing Gel which is used to remove residual capsaicin from the skin after treatment. Cleansing Gel consists of the following ingredients: butylated hydroxyanisole, carbomer copolymer, edetate disodium, polyethylene glycol, purified water, and sodium hydroxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Capsaicin is an agonist for the transient receptor potential vanilloid 1 receptor (TRPV1), which is an ion channel-receptor complex expressed on nociceptive nerve fibers in the skin. Topical administration of capsaicin causes an initial enhanced stimulation of the TRPV1-expressing cutaneous nociceptors that may be associated with painful sensations. This is followed by pain relief thought to be mediated by a reduction in TRPV1-expressing nociceptive nerve endings [ see Clinical Pharmacology (12.2) ]. Over the course of several months, there may be a gradual re-emergence of painful neuropathy thought to be due to TRPV1 nerve fiber reinnervation of the treated area.
12.2 Pharmacodynamics
Two studies evaluated the pharmacodynamic effects of Qutenza on sensory function and epidermal nerve fiber (ENF) density in healthy volunteers. Consistent with the known pharmacodynamic effects of capsaicin on TRPV1-expressing nociceptive nerve endings, reduced ENF density and minor changes in cutaneous nociceptive function (heat detection and sharp sensation) were noted one week after exposure to Qutenza. ENF density reduction and sensory changes were fully reversible.
12.3 Pharmacokinetics
Pharmacokinetic data in humans showed transient, low (< 5 ng/mL) systemic exposure to capsaicin in about one third of PHN patients following 60-minute applications of Qutenza. The highest plasma concentration of capsaicin detected was 4.6 ng/mL and occurred immediately after Qutenza removal. Most quantifiable levels were observed at the time of Qutenza removal and were below the limit of quantitation 3 to 6 hours after Qutenza removal. No detectable levels of metabolites were observed in any subject.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Mutagenesis
Capsaicin was not mutagenic in the Ames, mouse micronucleus and chromosomal aberration in human peripheral blood lymphocytes assays. As with other catechol-containing compounds (e.g., dopamine), capsaicin showed a weak mutagenic response in the mouse lymphoma assay.
Impairment of Fertility
A fertility and reproductive toxicology study was conducted in rats with exposure to Qutenza patches daily for 3 hours/day beginning 28 days before cohabitation, through cohabitation and continuing through the day before sacrifice (approximately 49 days of treatment). The results revealed a statistically significant reduction in the number and percent of motile sperm. Sperm motility obtained from the vas deferens was reduced in all capsaicin treatment groups (16, 24 and 32 mg capsaicin patch/rat/day). Though a "no effect" level was not determined, dose levels used in the study correspond to a 13- to 28-fold exposure margin over the mean C max associated with the maximal human recommended dose. Sperm counts were reduced in the vas deferens or cauda epididymis in the 24 and 32 mg capsaicin patch/rat/day dose groups (79 and 69%, respectively) compared to the placebo patch treated control group; however, these reductions did not adversely affect fertility. As this animal model has a large excess of sperm generating capacity relative to the threshold necessary for fertilization, the lack of an effect on fertility in this species is of unknown significance for human risk assessment.
-
14 CLINICAL STUDIES
14.1 Postherpetic Neuralgia
The efficacy of Qutenza, was established in two 12-week, double-blind, randomized, dose-controlled, multicenter studies. These studies enrolled patients with postherpetic neuralgia (PHN) persisting for at least 6 months following healing of herpes zoster rash and a baseline score of 3-9 on an 11-point Numerical Pain Rating Scale (NPRS) ranging from 0 (no pain) to 10 (worst possible pain). Qutenza and a control patch were each applied as a single 60-minute application. The control used in these studies looked similar to Qutenza but contained a low concentration of the active ingredient, capsaicin (3.2 mcg/cm 2, 0.04% w/w) to retain blinding regarding the known application site reactions of capsaicin (such as burning and erythema). The baseline mean pain scores across the 2 studies was approximately 6.0. Patients who entered the study on stable doses of pain-control medications were required to keep dosing stable throughout the duration of the study. Approximately half of the patients were taking concomitant medications including anticonvulsants, non-SSRI antidepressants, or opioids for their PHN at study entry. Prior to study patch application a topical anesthetic was applied to the treatment area for 60 minutes. Patients were permitted to use local cooling and additional analgesic medications for treatment-related discomfort as needed through Day 5. Patients recorded their pain daily in a diary.
PHN Study 1: In this 12-week study, the Qutenza group demonstrated a greater reduction in pain compared to the Control group during the primary assessment at Week 8. The percent change in average pain from baseline to Week 8 was -18% (±2%) for the low-dose control and -29% (±2%) for Qutenza.
For various degrees of improvement in pain from baseline to study endpoint, Figure 2 shows the fraction of patients achieving that degree of improvement. The figure is cumulative, so that patients whose change from baseline is, for example, 50%, are also included at every level of improvement below 50%. Patients who did not complete the study through Week 12 or who showed no improvement at Week 12 were assigned 0% improvement. Some patients experienced a decrease in pain as early as Week 1, which persisted throughout the study. The proportion of patients experiencing ≥ 30% reduction in pain intensity from baseline for each week through Week 12 is shown in Figure 3.
FIGURE 2:
Patients Achieving Various Percentages of Reduction in Pain Intensity at
Week 12 – Study 1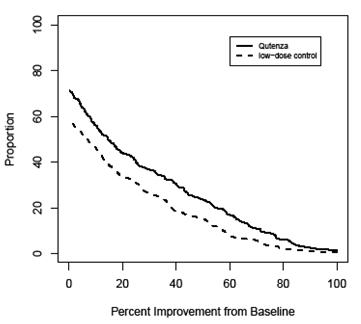
FIGURE 3:
Weekly Proportion of Patients Achieving ≥ 30% Pain
Intensity Reduction – Study 1*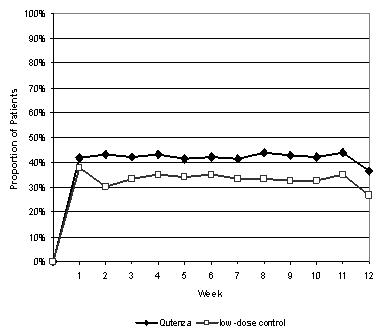
PHN Study 2: In this 12-week study the Qutenza group demonstrated a greater reduction in pain compared to the Control group during the primary assessment at Week 8. The percent change in average pain from baseline to Week 8 was -26% (±2%) for the low-dose control and -33% (±2%) for Qutenza.
For various degrees of improvement in pain from baseline to study endpoint, Figure 4 shows the fraction of patients achieving that degree of improvement. The figure is cumulative, so that patients whose change from baseline is, for example, 50%, are also included at every level of improvement below 50%. Patients who did not complete the study through Week 12 or who showed no improvement at Week 12 were assigned 0% improvement. Some patients experienced a decrease in pain as early as Week 1, which persisted throughout the study. The proportion of patients achieving ≥ 30% reduction in pain intensity from baseline for each week through Week 12 is shown in Figure 5.
FIGURE 4:
Patients Achieving Various Percentages of Reduction in Pain Intensity at
Week 12 – Study 2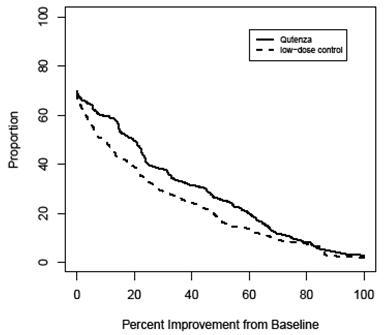
FIGURE 5:
Weekly Proportion of Patients Achieving ≥ 30% Pain
Intensity Reduction – Study 2*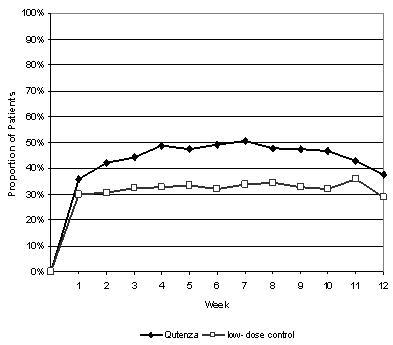
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Qutenza (capsaicin) 8% patch is a single-use patch stored in a sealed pouch (NDC: 10144-920-00).
Each individual patch is printed with "capsaicin 8%".
Cleansing Gel is provided in a 50 g tube.
Qutenza is available in the following presentations:
Carton of 1 patch and 50 g tube of Cleansing Gel
(NDC: 10144-928-01).
Carton of 2 patches and 50 g tube of Cleansing Gel
(NDC: 10144-929-01).
16.2 Storage
Store carton between 20° to 25°C (68° to 77°F). Excursions between 15°C and 30°C (59°F and 86°F) are allowed.
Keep the patch in the sealed pouch until immediately before use.
16.3 Handling and Disposal
Qutenza contains capsaicin capable of producing severe irritation of eyes, skin, respiratory tract and mucous membranes. Do not dispense Qutenza to patients for self-administration. It is critical that health care professionals who administer Qutenza have completely familiarized themselves with proper dosing, handling, and disposal procedures before handling Qutenza to avoid accidental or inadvertent capsaicin exposure to themselves or others [ see Dosage and Administration (2) ].
- Do not touch Qutenza, treatment areas, and all used supplies or other materials placed in contact with the treatment area without wearing nitrile gloves.
- Wear nitrile gloves at all times while handling Qutenza and cleaning treatment areas. Do NOT use latex gloves.
- Do not hold Qutenza near eyes or mucous membranes.
- Immediately after use, dispose of used and unused patches, patch clippings, unused Cleansing Gel and associated treatment supplies in accordance with local biomedical waste procedures.
-
17 PATIENT COUNSELING INFORMATION
- Inform patients that exposure of the skin to Qutenza may result in transient erythema and burning sensation. Instruct patients not to touch the patch and that if they accidentally touch the Qutenza patch it may burn and/or sting.
- Instruct patients that if irritation of eyes or airways occurs, or if any of the side effects become severe, to notify their doctor immediately.
- Inform patients that the treated area may be sensitive to heat (e.g., hot showers/bath, direct sunlight, vigorous exercise) for a few days following treatment.
- Inform patients that they may be given medication to treat acute pain during and after the Qutenza application procedure. Some of these medications, such as opioids, may affect the ability to perform potentially hazardous activities such as driving or operating machinery.
- Inform patients that as a result of treatment-related increases in pain, small transient increases in blood pressure may occur during and shortly after Qutenza treatment and that blood pressure will be monitored during the treatment procedure. Instruct patients to inform the physician if they have experienced any recent cardiovascular event
- Instruct patients to notify their physician if they are pregnant or breast feeding.
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - Pouch Label
NDC: 10144-920-00
Qutenza®
(capsaicin) 8% patchRx only. For topical use only.
One single-use patch (179 mg capsaicin)To be opened by a healthcare
professional only.Use of nitrile gloves is required.
Do not use latex gloves.Keep Qutenza ® in the sealed pouch until immediately before use.
Dosage/Instructions for Use: See Full Prescribing Information.
After removal of Qutenza, apply Cleansing Gel for one minute and them remove it with a dry wipe.
Qutenza ® contains capsaicin and may cause irritation.ACORDA®
THERAPEUTICS0713920SQPP-0

-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 50g Tube Label
Cleansing Gel for Qutenza ®
(capsaicin) 8% patch50g
FOR TOPICAL USE - AFTER ADMINISTRATION OF QUTENZA ®PATCH ONLY
Instructions for Use: See Full Prescribing Information for Qutenza ®.
Use of nitrile gloves is required. Do not use latex gloves.Store between 20°C to 25°C (68°F to 77°F). Excursions between 15°C
and 30°C (59°F and 86°F) are allowed.
Ingredients:
Butylated hydroxyanisole, carbomer copolymer, edetate disodium,
polyethylene glycol, purified water, and sodium hydroxide.Rev. July - 2013
Manufactured for Acorda Therapeutics, Inc., Ardsley, NY 10502, USA
by Contract Pharmaceuticals Limited Canada (CPL)
Mississauga, ON L5N 6L6, Canada©Acorda Therapeutics, Inc. 2013
MADE IN CANADAACORDA®
THERAPEUTICS0713-SQG-0
Patent Pending

-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 1 Pouch Kit Carton
NDC: 10144-928-01
1 PatchOne single-use patch (179 mg capsaicin)
One single-use 50 g tube of Cleansing GelQutenza®
(capsaicin) 8% patchRx only. For topical use only.
Dosage/Instructions for Use: See Full Prescribing Information.
Only a healthcare professional should open the Qutenza ® carton or pouch.
Use of nitrile gloves is required when handling Qutenza ®. Do not use latex gloves. Qutenza ®contains capsaicin and
may cause irritation.Qutenza ® should be administered by a physician or healthcare professional under the close supervision of a physician.
ACORDA®
THERAPEUTICSManufactured for Acorda Therapeutics, Inc., Ardsley, NY 10502, USA by Lohmann Therapie-Systeme AG (LTS) Andernach, Germany © Acorda Therapeutics, Inc. 2013
For questions about Qutenza ® call 1-877-900-6479 Rev. July 2013 0713928SQG1-0
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 2 Pouch Kit Carton
NDC: 10144-929-01
2 PatchesTwo single-use patches (179 mg capsaicin)
One single-use 50 g tube of Cleansing GelQutenza®
(capsaicin) 8% patchRx only. For topical use only.
Dosage/Instructions for Use: See Full Prescribing Information.
Only a healthcare professional should open the Qutenza ® carton or pouch.
Use of nitrile gloves is required when handling Qutenza ®. Do not use latex gloves. Qutenza ®contains capsaicin and
may cause irritation.Qutenza ® should be administered by a physician or healthcare professional under the close supervision of a physician.
ACORDA®
THERAPEUTICSManufactured for Acorda Therapeutics, Inc., Ardsley, NY 10502, USA by Lohmann Therapie-Systeme AG (LTS) Andernach, Germany © Acorda Therapeutics, Inc. 2013
For questions about Qutenza ® call 1-877-900-6479 Rev. July 2013 0713929SQG2-0
-
INGREDIENTS AND APPEARANCE
QUTENZA
capsaicin kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 10144-928 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 10144-928-01 1 in 1 CARTON 08/12/2013 11/30/2020 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 PATCH 280 cm2 Part 2 1 TUBE 50 g Part 1 of 2 QUTENZA
capsaicin patchProduct Information Item Code (Source) NDC: 10144-920 Route of Administration CUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAPSAICIN (UNII: S07O44R1ZM) (CAPSAICIN - UNII:S07O44R1ZM) CAPSAICIN 640 ug in 1 cm2 Inactive Ingredients Ingredient Name Strength DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) DIMETHICONE (UNII: 92RU3N3Y1O) ETHYLCELLULOSES (UNII: 7Z8S9VYZ4B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 10144-920-00 1 in 1 POUCH 1 280 cm2 in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022395 08/12/2013 11/30/2020 Part 2 of 2 CLEANSING
cleansing (cold creams, cleansing lotions, liquids, and pads) gelProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) INGR EDETATE DISODIUM (UNII: 7FLD91C86K) INGR POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) INGR WATER (UNII: 059QF0KO0R) INGR SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 50 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 08/12/2013 11/30/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022395 08/12/2013 11/30/2020 QUTENZA
capsaicin kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 10144-929 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 10144-929-01 1 in 1 CARTON 08/12/2013 11/30/2020 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 2 PATCH 560 cm2 Part 2 1 TUBE 50 g Part 1 of 2 QUTENZA
capsaicin patchProduct Information Route of Administration CUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAPSAICIN (UNII: S07O44R1ZM) (CAPSAICIN - UNII:S07O44R1ZM) CAPSAICIN 640 ug in 1 cm2 Inactive Ingredients Ingredient Name Strength DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) DIMETHICONE (UNII: 92RU3N3Y1O) ETHYLCELLULOSES (UNII: 7Z8S9VYZ4B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 POUCH 1 280 cm2 in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022395 08/12/2013 11/30/2020 Part 2 of 2 CLEANSING
cleansing (cold creams, cleansing lotions, liquids, and pads) gelProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) INGR EDETATE DISODIUM (UNII: 7FLD91C86K) INGR POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) INGR WATER (UNII: 059QF0KO0R) INGR SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 50 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 08/12/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022395 08/12/2013 11/30/2020 Labeler - Acorda Therapeutics, Inc. (963845136) Establishment Name Address ID/FEI Business Operations Sharp Corporation 002346625 relabel(10144-920, 10144-928, 10144-929) , repack(10144-920, 10144-928, 10144-929) Establishment Name Address ID/FEI Business Operations Sharp Corporation 143696495 relabel(10144-920, 10144-928, 10144-929) , repack(10144-920, 10144-928, 10144-929) Establishment Name Address ID/FEI Business Operations Contract Pharmaceuticals Limited Canada - Lab Svcs (CPL) 202733655 analysis(10144-928, 10144-929) Establishment Name Address ID/FEI Business Operations SGS Life Science Services 203668041 analysis(10144-928, 10144-929) Establishment Name Address ID/FEI Business Operations Exova Canada Inc. 243681538 analysis(10144-928, 10144-929) Establishment Name Address ID/FEI Business Operations Contract Pharmaceuticals Limited Canada (CPL) 248761249 manufacture(10144-928, 10144-929) Establishment Name Address ID/FEI Business Operations SGS Institut Fresenius GmbH 317219699 analysis(10144-920) Establishment Name Address ID/FEI Business Operations Lohmann Therapie-Systeme AG (LTS) (Germany) 342693590 manufacture(10144-920) Establishment Name Address ID/FEI Business Operations SGS Life Science Services 808308303 analysis(10144-920, 10144-928, 10144-929)
Trademark Results [Qutenza]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 QUTENZA 77144310 3719009 Live/Registered |
AVERITAS PHARMA, INC. 2007-03-29 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.