VANCOMYCIN HYDROCHLORIDE capsule
vancomycin hydrochloride by
Drug Labeling and Warnings
vancomycin hydrochloride by is a Prescription medication manufactured, distributed, or labeled by Heritage Pharmaceuticals Inc. d/b/a Avet Pharmaceuticals Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use VANCOMYCIN HYDROCHLORIDE CAPSULES safely and effectively. See full prescribing information for VANCOMYCIN HYDROCHLORIDE CAPSULES.
VANCOMYCIN HYDROCHLORIDE capsules, for oral use
Initial U.S. Approval: 1986INDICATIONS AND USAGE
Vancomycin hydrochloride capsules are glycopeptide antibacterial indicated in adult and pediatric patients (less than 18 years of age) for the treatment of: (1) (1)
- Clostridioides difficile-associated diarrhea
- Enterocolitis caused by Staphylococcus aureus (including methicillin-resistant strains)
Limitations of Use: (1) (5.1) (1)
- Parenteral administration of vancomycin is not effective for the above infections; therefore, vancomycin hydrochloride capsules must be given orally for these infections.
- Orally administered vancomycin hydrochloride capsules are not effective for other types of infections.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of vancomycin hydrochloride capsules and other antibacterial drugs, vancomycin hydrochloride capsules should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. (1) (1)
DOSAGE AND ADMINISTRATION
-
C. difficile-associated diarrhea:
- Adult Patients (18 years of age or greater): 125 mg orally 4 times daily for 10 days. (2.1)
- Pediatric Patients (less than 18 years of age): 40 mg/kg in 3 or 4 divided doses for 7 to 10 days. The total daily dosage should not exceed 2 g. (2.2)
- Staphylococcal enterocolitis:
- Adult Patients (18 years of age or greater): 500 mg to 2 g orally in 3 or 4 divided doses for 7 to 10 days. (2.1)
- Pediatric Patients (less than 18 years of age): 40 mg/kg in 3 or 4 divided doses for 7 to 10 days. The total daily dosage should not exceed 2 g. (2.2)
DOSAGE FORMS AND STRENGTHS
125 mg capsules and 250 mg capsules (3) (3)
CONTRAINDICATIONS
Hypersensitivity to vancomycin (4) (4)
WARNINGS AND PRECAUTIONS
- Vancomycin must be given orally for treatment of staphylococcal enterocolitis and C. difficile-associated diarrhea. Orally administered Vancomycin capsules are not effective for other types of infections. (5.1)
- Clinically significant serum concentrations have been reported in some patients who have taken multiple oral doses of vancomycin for active C. difficile-associated diarrhea. Monitoring of serum concentrations may be appropriate in some instances. (5.2)
- Nephrotoxicity has occurred following oral vancomycin therapy and can occur either during or after completion of therapy. The risk is increased in geriatric patients (5.3) Monitor renal function.
- Ototoxicity has occurred in patients receiving vancomycin (5.4) Assessment of auditory function may be appropriate in some instances.
- Severe Dermatologic Reactions: Discontinue Vancomycin hydrochloride capsules at the first appearance of skin rashes, mucosal lesions, or blisters. (5.5)
- Prescribing vancomycin in the absence of a proven or strongly suspected bacterial infection is unlikely to provide benefit to the patient and increases the risk of the development of drug resistant bacteria. (5.6)
ADVERSE REACTIONS
USE IN SPECIFIC POPULATIONS
Geriatrics: In patients >65 years of age, including those with normal renal function prior to treatment, renal function should be monitored during and following treatment with vancomycin to detect potential vancomycin induced nephrotoxicity. (5.3) (6.1) (8.5) (14.1) (8)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Adults
2.2 Pediatric Patients (less than 18 years of age)
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Oral Use Only
5.2 Potential for Systemic Absorption
5.3 Nephrotoxicity
5.4 Ototoxicity
5.5 Severe Dermatologic Reactions
5.6 Development of Drug-Resistant Bacteria
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Diarrhea Associated with Clostridioides difficile
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Vancomycin hydrochloride capsules are indicated for the treatment of Clostridioides difficile-associated diarrhea. Vancomycin hydrochloride capsules are also used for the treatment of enterocolitis caused by Staphylococcus aureus (including methicillin-resistant strains) in adult and pediatric patients less than 18 years of age.
Limitations of Use
- Parenteral administration of vancomycin is not effective for the above infections; therefore, vancomycin hydrochloride capsules must be given orally for these infections.
- Orally administered vancomycin hydrochloride capsules are not effective for other types of infections.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of vancomycin hydrochloride capsules and other antibacterial drugs, vancomycin hydrochloride capsules should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
-
2 DOSAGE AND ADMINISTRATION
2.1 Adults
Vancomycin hydrochloride capsules are used in treating C. difficile-associated diarrhea and staphylococcal enterocolitis.
- C. difficile-associated diarrhea: The recommended dose is 125 mg administered orally 4 times daily for 10 days.
- Staphylococcal enterocolitis: Total daily dosage is 500 mg to 2 g administered orally in 3 or 4 divided doses for 7 to 10 days.
-
3 DOSAGE FORMS AND STRENGTHS
Vancomycin hydrochloride capsules, USP 125 mg* have a grey cap and pink body imprinted with "SAL" on the cap and "729" on the body in black ink.
Vancomycin hydrochloride capsules, USP 250 mg* have a brown cap and brown body imprinted with "SAL" on the cap and "730" on the body in white ink.
*Equivalent to vancomycin.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Oral Use Only
Vancomycin hydrochloride capsules for the treatment of colitis is for oral use only and is not systemically absorbed. Vancomycin hydrochloride capsules must be given orally for treatment of staphylococcal enterocolitis and Clostridioides difficile-associated diarrhea. Orally administered Vancomycin hydrochloride capsules are not effective for other types of infections.
Parenteral administration of vancomycin is not effective for treatment of staphylococcal enterocolitis and C. difficile-associated diarrhea. If parenteral vancomycin therapy is desired, use an intravenous preparation of vancomycin and consult the package insert accompanying that preparation.
5.2 Potential for Systemic Absorption
Clinically significant serum concentrations have been reported in some patients who have taken multiple oral doses of vancomycin for active C. difficile-associated diarrhea. Some patients with inflammatory disorders of the intestinal mucosa also may have significant systemic absorption of vancomycin. These patients may be at risk for the development of adverse reactions associated with higher doses of vancomycin hydrochloride; therefore, monitoring of serum concentrations of vancomycin may be appropriate in some instances, e.g., in patients with renal insufficiency and/or colitis or in those receiving concomitant therapy with an aminoglycoside antibiotic.
5.3 Nephrotoxicity
Nephrotoxicity (e.g., reports of renal failure, renal impairment, blood creatinine increased) has occurred following oral vancomycin hydrochloride therapy in randomized controlled clinical studies, and can occur either during or after completion of therapy. The risk of nephrotoxicity is increased in patients >65 years of age [see Adverse Reactions (6.1) and Use in Specific Populations (8.5)].
In patients >65 years of age, including those with normal renal function prior to treatment, renal function should be monitored during and following treatment with vancomycin hydrochloride capsules to detect potential vancomycin induced nephrotoxicity.
5.4 Ototoxicity
Ototoxicity has occurred in patients receiving vancomycin. It may be transient or permanent. It has been reported mostly in patients who have been given excessive intravenous doses, who have an underlying hearing loss, or who are receiving concomitant therapy with another ototoxic agent, such as an aminoglycoside. Serial tests of auditory function may be helpful in order to minimize the risk of ototoxicity [see Adverse Reactions (6.2)].
5.5 Severe Dermatologic Reactions
Severe dermatologic reactions such as toxic epidermal necrolysis (TEN), Stevens-Johnson syndrome(SJS), drug reaction with eosinophilia and systemic symptoms (DRESS), acute generalized exanthematous pustulosis (AGEP), and linear IgA bullous dermatosis (LABD) have been reported in association with the use of vancomycin. Cutaneous signs or symptoms reported include skin rashes, mucosal lesions, and blisters.
Discontinue vancomycin hydrochloride capsules at the first appearance of signs and symptoms of TEN, SJS, DRESS, AGEP, or LABD.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to vancomycin hydrochloride in 260 adult subjects in two Phase 3 clinical trials for the treatment of diarrhea associated with C. difficile. In both trials, subjects received vancomycin hydrochloride 125 mg orally four times daily. The mean duration of treatment was 9.4 days. The median age of patients was 67, ranging between 19 and 96 years of age. Patients were predominantly Caucasian (93%) and 52% were male.
Adverse reactions occurring in ≥ 5% of vancomycin hydrochloride-treated subjects are shown in Table 1. The most common adverse reactions associated with vancomycin hydrochloride (≥ 10%) were nausea, abdominal pain, and hypokalemia.
Table 1: Common (≥ 5%) Adverse Reactionsa for Vancomycin Hydrochloride Reported in Clinical Trials for Treatment of Diarrhea Associated with C. difficile aAdverse reaction rates were derived from the incidence of treatment-emergent adverse events
System/Organ Class Adverse Reaction Vancomycin hydrochloride % (N=260) Gastrointestinal disorders Nausea 17 Abdominal pain 15 Vomiting 9 Diarrhea 9 Flatulence 8 General disorders and administration site conditions Pyrexia 9 Edema peripheral 6 Fatigue 5 Infections and infestations Urinary tract infection 8 Metabolism and nutrition disorders Hypokalemia 13 Musculoskeletal and connective tissue disorders Back pain 6 Nervous system disorders Headache 7 Nephrotoxicity (e.g., reports of renal failure, renal impairment, blood creatinine increased) occurred in 5% of subjects treated with vancomycin hydrochloride. Nephrotoxicity following vancomycin hydrochloride typically first occurred within one week after completion of treatment (median day of onset was Day 16).
Nephrotoxicity following vancomycin hydrochloride occurred in 6% of subjects >65 years of age and 3% of subjects ≤65 years of age [see Warnings and Precautions (5.3)].
The incidences of hypokalemia, urinary tract infection, peripheral edema, insomnia, constipation, anemia, depression, vomiting, and hypotension were higher among subjects >65 years of age than in subjects ≤ 65 years of age [see Use in Specific Populations (8.5)].
Discontinuation of study drug due to adverse events occurred in 7% of subjects treated with vancomycin hydrochloride. The most common adverse events leading to discontinuation of vancomycin hydrochloride were C. difficile colitis (<1%), nausea (<1%), and vomiting (<1%).
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of vancomycin hydrochloride. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Ototoxicity: Cases of hearing loss associated with intravenously administered vancomycin have been reported. Most of these patients had kidney dysfunction or a preexisting hearing loss or were receiving concomitant treatment with an ototoxic drug [see Warnings and Precautions (5.4)]. Vertigo, dizziness, and tinnitus have been reported.
Skin and Subcutaneous Tissue Disorders: Severe dermatologic reactions such as toxic epidermal necrolysis (TEN), Stevens-Johnson syndrome (SJS), drug reaction with eosinophilia and systemic symptoms (DRESS), acute generalized exanthematous pustulosis (AGEP), and linear IgA bullous dermatosis (LABD) [see Warnings and Precautions (5.5)], rashes (including exfoliative dermatitis).
Hematopoietic: Reversible neutropenia, usually starting 1 week or more after onset of intravenous therapy with vancomycin or after a total dose of more than 25 g, has been reported for several dozen patients. Neutropenia appears to be promptly reversible when vancomycin is discontinued. Thrombocytopenia has been reported.
Miscellaneous: Patients have been reported to have had anaphylaxis, drug fever, chills, nausea, eosinophilia and cases of vasculitis in association with the administration of vancomycin.
A condition has been reported that is similar to the IV–induced syndrome with symptoms consistent with anaphylactoid reactions, including hypotension, wheezing, dyspnea, urticaria, pruritus, flushing of the upper body ("vancomycin infusion reaction"), pain and muscle spasm of the chest and back. These reactions usually resolve within 20 minutes but may persist for several hours.
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Systemic absorption of vancomycin is low following oral administration of vancomycin hydrochloride; however, absorption may vary depending on various factors [see Clinical Pharmacology (12.3)].
There are no available data on vancomycin use in pregnant women to assess a risk of major birth defects or miscarriage. Available published data on intravenous vancomycin use in pregnancy during the second and third trimesters have not shown an association with adverse maternal or fetal outcomes (see Data).
Vancomycin did not show adverse developmental effects when administered intravenously to pregnant rats and rabbits during organogenesis at doses less than or equal to the recommended maximum human dose (see Data).
Data
Human Data
There are no available data on first trimester use of vancomycin in pregnant women to assess a risk of major birth defects or miscarriage.
A published study evaluated hearing loss and nephrotoxicity in infants of 10 pregnant intravenous drug users treated with intravenous vancomycin for suspected or documented methicillin-resistant Staphylococcal aureus in the second or third trimester. The comparison groups were 10 uninfected non-intravenous drug-dependent patients who received no treatment and 10 uninfected untreated intravenous drug-dependent patients. No infant in the vancomycin exposed group had abnormal sensorineural hearing at 3 months of age or nephrotoxicity.
A published prospective study assessed outcomes in 55 pregnant women with a positive Group B streptococcus culture and a high-risk penicillin allergy with resistance to clindamycin or unknown sensitivity who were administered intravenous vancomycin at the time of delivery. Vancomycin dosing ranged from the standard dose of 1 g intravenously every 12 hours to a dose of 20 mg/kg intravenously every 8 hours (maximum individual dose 2 g). No major adverse reactions were recorded either in the mothers or their newborns. None of the newborns had sensorineural hearing loss. Neonatal renal function was not examined, but all of the newborns were discharged in good condition.
Animal Data
Vancomycin did not cause fetal malformation when administered intravenously during organogenesis to pregnant rats (gestation days 6 to 15) and rabbits (gestation days 6 to 18) at the equivalent recommended maximum human dose of 200 mg/kg/day to rats or 120 mg/kg/day to rabbits. No effects on fetal weight or development were seen in rats at the highest dose tested or in rabbits given 80 mg/kg/day (approximately 1 and 0.8 times the recommended maximum human dose based on body surface area). Maternal toxicity was observed in rats (at doses 120 mg/kg and above) and rabbits (at 80 mg/kg and above).
8.2 Lactation
There are no data on the presence of vancomycin in human milk, the effects on the breastfed infant, or the effect on milk production following oral administration. Systemic absorption of vancomycin is low following oral administration of vancomycin hydrochloride [see Clinical Pharmacology (12.3)]; therefore, it is unlikely to result in clinically relevant exposure in breastfeeding infants. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for vancomycin hydrochloride and any potential adverse effects on the breastfed infant from vancomycin hydrochloride or from the underlying maternal condition.
8.4 Pediatric Use
Vancomycin hydrochloride is indicated in pediatric patients less than 18 years of age for the treatment of C. difficile-associated diarrhea and enterocolitis caused by S. aureus (including methicillin-resistant strains) [see Indications and Usage (1) and Dosage and Administration (2.2)].
8.5 Geriatric Use
In clinical trials, 54% of vancomycin hydrochloride-treated subjects were >65 years of age. Of these, 40% were between the ages of >65 and 75, and 60% were >75 years of age.
Clinical studies with vancomycin hydrochloride in diarrhea associated with Clostridioides difficile have demonstrated that geriatric subjects are at increased risk of developing nephrotoxicity following treatment with oral vancomycin hydrochloride, which may occur during or after completion of therapy. In patients >65 years of age, including those with normal renal function prior to treatment, renal function should be monitored during and following treatment with vancomycin hydrochloride to detect potential vancomycin induced nephrotoxicity [see Warnings and Precautions (5.3), Adverse Reactions (6.1), and Clinical Studies (14.1)].
Patients >65 years of age may take longer to respond to therapy compared to patients ≤65 years of age [see Clinical Studies (14.1)]. Clinicians should be aware of the importance of appropriate duration of vancomycin hydrochloride treatment in patients >65 years of age and not discontinue or switch to alternative treatment prematurely.
-
10 OVERDOSAGE
Supportive care is advised, with maintenance of glomerular filtration. Vancomycin is poorly removed by dialysis. Hemofiltration and hemoperfusion with polysulfone resin have been reported to result in increased vancomycin clearance.
To obtain current information about the treatment of overdose, contact a certified Poison Control Center (1-800-222-1222 or www.poison.org). In managing overdosage, consider the possibility of multiple drug overdoses, interaction among drugs, and unusual drug kinetics.
-
11 DESCRIPTION
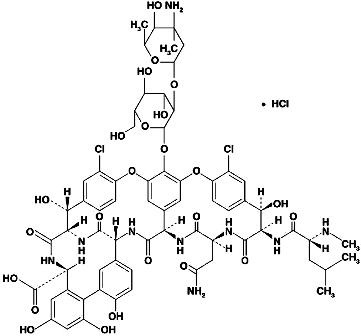
Vancomycin hydrochloride capsules, USP for oral administration contain chromatographically purified vancomycin hydrochloride, a tricyclic glycopeptide antibiotic derived from Amycolatopsis orientalis (formerly Nocardia orientalis), which has the chemical formula C66H75Cl2N9O24HCl. The molecular weight of vancomycin hydrochloride is 1,485.73; 500 mg of the base is equivalent to 0.34 mmol.
Each capsule contains 125 mg vancomycin (equivalent to 128 mg vancomycin hydrochloride) or 250 mg vancomycin (equivalent to 256 mg vancomycin hydrochloride). Inactive ingredient includes polyethylene glycol.
The 125 mg capsule shell contains gelatin, FD&C Blue No. 1, D & C Red No. 28, D & C Yellow No.10, titanium dioxide, iron oxide red and iron oxide yellow. The capsules are printed with black ink. The black imprinting ink contains shellac, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, strong ammonia solution, black iron oxide and potassium hydroxide. The 250 mg capsule shell contains gelatin, black iron oxide, iron oxide red, iron oxide yellow and titanium dioxide. The capsules are printed with white ink. The white imprinting ink contains shellac, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, strong ammonia solution, potassium hydroxide and titanium dioxide.
Vancomycin hydrochloride has the structural formula:
-
12 CLINICAL PHARMACOLOGY
12.3 Pharmacokinetics
Vancomycin is poorly absorbed after oral administration. During multiple dosing of 250 mg every 8 hours for 7 doses, fecal concentrations of vancomycin in volunteers exceeded 100 mg/kg in the majority of samples. No blood concentrations were detected and urinary recovery did not exceed 0.76%. In anephric subjects with no inflammatory bowel disease who received vancomycin oral solution 2 g for 16 days, blood concentrations of vancomycin were less than or equal to 0.66 mcg/mL in 2 of 5 subjects. No measurable blood concentrations were attained in the other 3 subjects. Following doses of 2 g daily, concentrations of drug were >3,100 mg/kg in the feces and <1 mcg/mL in the serum of subjects with normal renal function who had C. difficile-associated diarrhea. After multiple-dose oral administration of vancomycin, measurable serum concentrations may occur in patients with active C. difficile-associated diarrhea, and, in the presence of renal impairment, the possibility of accumulation exists. It should be noted that the total systemic and renal clearances of vancomycin are reduced in the elderly [see Use in Specific Populations (8.5)].
12.4 Microbiology
The bactericidal action of vancomycin against Staphylococcus aureus and the vegetative cells of Clostridioides difficile results primarily from inhibition of cell-wall biosynthesis. In addition, vancomycin alters bacterial-cell-membrane permeability and RNA synthesis.
Resistance
Staphylococcus aureus
S. aureus isolates with vancomycin minimal inhibitory concentrations (MICs) as high as 1,024 mcg/mL have been reported.
The exact mechanism of this resistance is not clear but is believed to be due to cell wall thickening and potentially the transfer of genetic material.
Clostridioides difficile
Isolates of C. difficile generally have vancomycin MICs of <1 mcg/mL, however vancomycin MICs ranging from 4 mcg/mL to 16 mcg/mL have been reported. The mechanism which mediates C. difficile's decreased susceptibility to vancomycin has not been fully elucidated.
Vancomycin has been shown to be active against most isolates of the following microorganisms, both in vitro and in clinical infections [see Indications and Usage (1)].
Gram-positive bacteria
Staphylococcus aureus (including methicillin-resistant isolates) associated with enterocolitis.
Anaerobic gram-positive bacteria
Clostridioides difficile isolates associated with C. difficile associated diarrhea.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term carcinogenesis studies in animals have been conducted.
At concentrations up to 1,000 mcg/mL, vancomycin had no mutagenic effect in vitro in the mouse lymphoma forward mutation assay or the primary rat hepatocyte unscheduled DNA synthesis assay. The concentrations tested in vitro were above the peak plasma vancomycin concentrations of 20 to 40 mcg/mL usually achieved in humans after slow infusion of the maximum recommended dose of 1 g. Vancomycin had no mutagenic effect in vivo in the Chinese hamster sister chromatid exchange assay (400 mg/kg IP) or the mouse micronucleus assay (800 mg/kg IP).
No definitive fertility studies have been conducted.
-
14 CLINICAL STUDIES
14.1 Diarrhea Associated with Clostridioides difficile
In two trials, vancomycin hydrochloride 125 mg orally four times daily for 10 days was evaluated in 266 adult subjects with C. difficile-associated diarrhea (CDAD). Enrolled subjects were 18 years of age or older and received no more than 48 hours of treatment with oral vancomycin hydrochloride or oral/intravenous metronidazole in the 5 days preceding enrollment. CDAD was defined as ≥3 loose or watery bowel movements within the 24 hours preceding enrollment, and the presence of either C. difficile toxin A or B, or pseudomembranes on endoscopy within the 72 hours preceding enrollment. Subjects with fulminant C. difficile disease, sepsis with hypotension, ileus, peritoneal signs or severe hepatic disease were excluded.
Efficacy analyses were performed on the Full Analysis Set (FAS), which included randomized subjects who received at least one dose of vancomycin hydrochloride and had any post-dosing investigator evaluation data (N=259; 134 in Trial 1 and 125 in Trial 2).
The demographic profile and baseline CDAD characteristics of enrolled subjects were similar in the two trials. Vancomycin hydrochloride-treated subjects had a median age of 67 years, were mainly white (93%), and male (52%). CDAD was classified as severe (defined as 10 or more unformed bowel movements per day or WBC ≥15000/mm3) in 25% of subjects, and 47% were previously treated for CDAD.
Efficacy was assessed by using clinical success, defined as diarrhea resolution and the absence of severe abdominal discomfort due to CDAD, on Day 10. An additional efficacy endpoint was the time to resolution of diarrhea, defined as the beginning of diarrhea resolution that was sustained through the end of the prescribed active treatment period.
The results for clinical success for vancomycin hydrochloride-treated subjects in both trials are shown in Table 2.
Table 2: Clinical Success Rates (Full Analysis Set) Clinical Success Rate 95% Confidence Interval Vancomycin % (N) Trial 1 81.3 (134) (74.4, 88.3) Trial 2 80.8 (125) (73.5, 88.1) The median time to resolution of diarrhea was 5 days and 4 days in Trial 1 and Trial 2, respectively. For subjects older than 65 years of age, the median time to resolution was 6 days and 4 days in Trial 1 and Trial 2, respectively. In subjects with diarrhea resolution at end-of-treatment with vancomycin hydrochloride, recurrence of CDAD during the following four weeks occurred in 25 of 107 (23%) and 18 of 102 (18%) in Trial 1 and Trial 2, respectively.
Restriction Endonuclease Analysis (REA) was used to identify C. difficile baseline isolates in the BI group. In Trial 1, the vancomycin hydrochloride-treated subjects were classified at baseline as follows 31 (23%) with BI strain, 69 (52%) with non-BI strain, and 34 (25%) with unknown strain. Clinical success rates were 87% for BI strain, 81% for non-BI strain, and 76% for unknown strain. In subjects with diarrhea resolution at end-of treatment with vancomycin hydrochloride, recurrence of CDAD during the following four weeks occurred in 7 of 26 subjects with BI strain, 12 of 56 subjects with non-BI strain, and 6 of 25 subjects with unknown strain.
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Vancomycin hydrochloride capsules, USP are available in:
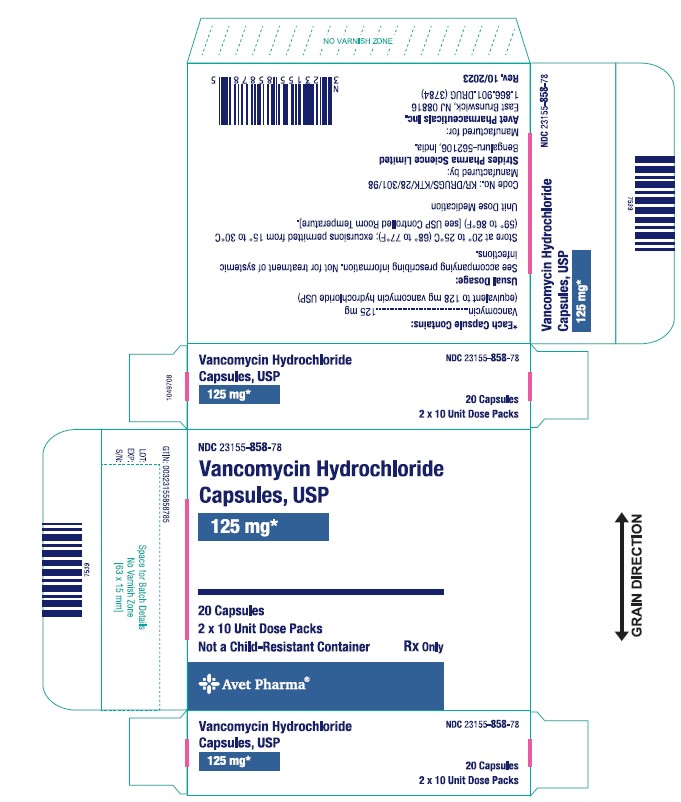
The 125 mg* capsules have a grey cap and pink body imprinted with "SAL" on the cap and "729" on the body in black ink.
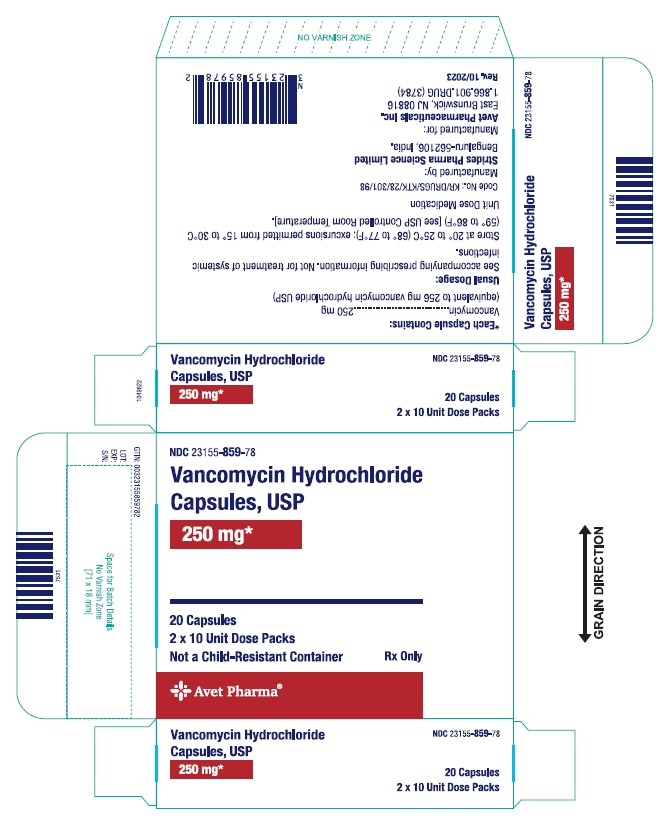
The 250 mg* capsules have a brown cap and brown body imprinted with "SAL" on the cap and "730" on the body in white ink.
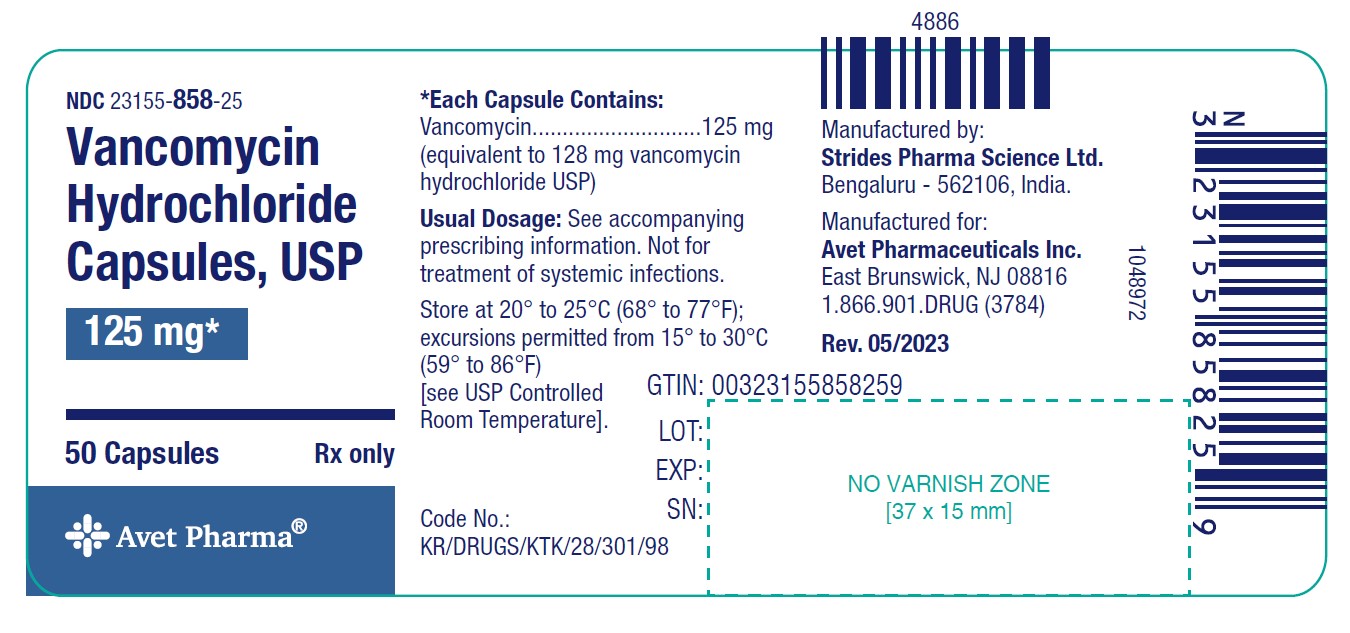
Strength Pack NDC number Vancomycin 125 mg Blister pack of 20 23155-858-78 Bottle pack of 50 23155-858-25 Vancomycin 250 mg Blister pack of 20 23155-859-78 Bottle pack of 50 23155-859-25 Store at 20° to 25°C (68° to 77°F); excursions permitted from 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
-
17 PATIENT COUNSELING INFORMATION
Advise patients about the signs and symptoms of serious skin manifestations. Instruct patients to stop taking vancomycin hydrochloride capsule immediately and promptly seek medical attention at the first signs or symptoms of skin rash, mucosal lesions or blisters [see Warnings and Precautions (5.5)].
Antibacterial Resistance
Patients should be counseled that antibacterial drugs including vancomycin hydrochloride should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When vancomycin hydrochloride is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by vancomycin hydrochloride or other antibacterial drugs in the future.
-
SPL UNCLASSIFIED SECTION
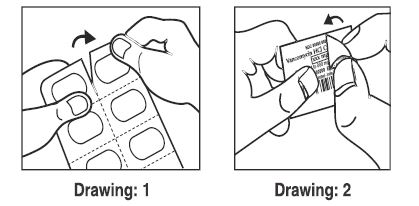
Following steps required for removal of capsule from blister and also refer below pictorial instructions for easy reference.
1. Collect one blister from the carton.
2. Hold the blister as such that it faces the printed blister foil side.
3. Blister shall be cut from the perforation marked on the blister.
4. Peel the printed paper where "PEEL TO OPEN" is given on the blister and take the capsule out.
Manufactured by:
Strides Pharma Science Limited
Bengaluru - 562106, India.
Manufactured for:
Avet Pharmaceuticals Inc.
East Brunswick, NJ 08816
1.866.901.DRUG (3784)

- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VANCOMYCIN HYDROCHLORIDE
vancomycin hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 23155-858 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VANCOMYCIN HYDROCHLORIDE (UNII: 71WO621TJD) (VANCOMYCIN - UNII:6Q205EH1VU) VANCOMYCIN 125 mg Inactive Ingredients Ingredient Name Strength GELATIN, UNSPECIFIED (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) D&C RED NO. 28 (UNII: 767IP0Y5NH) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) SHELLAC (UNII: 46N107B71O) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) FERROSOFERRIC OXIDE (UNII: XM0M87F357) ALCOHOL (UNII: 3K9958V90M) ISOPROPYL ALCOHOL (UNII: ND2M416302) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) AMMONIA (UNII: 5138Q19F1X) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) Product Characteristics Color GRAY (Grey cap and Pink body) Score no score Shape CAPSULE (capsule) Size 18mm Flavor Imprint Code SALand729 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 23155-858-25 50 in 1 BOTTLE; Type 0: Not a Combination Product 06/25/2023 08/31/2028 2 NDC: 23155-858-78 2 in 1 CARTON 06/25/2023 08/31/2027 2 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA065490 06/25/2023 08/31/2028 VANCOMYCIN HYDROCHLORIDE
vancomycin hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 23155-859 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VANCOMYCIN HYDROCHLORIDE (UNII: 71WO621TJD) (VANCOMYCIN - UNII:6Q205EH1VU) VANCOMYCIN 250 mg Inactive Ingredients Ingredient Name Strength GELATIN, UNSPECIFIED (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) SHELLAC (UNII: 46N107B71O) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) FERROSOFERRIC OXIDE (UNII: XM0M87F357) ALCOHOL (UNII: 3K9958V90M) ISOPROPYL ALCOHOL (UNII: ND2M416302) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) AMMONIA (UNII: 5138Q19F1X) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) Product Characteristics Color BROWN (Brown Cap and Brown Body) Score no score Shape CAPSULE (capsule) Size 18mm Flavor Imprint Code SALand730 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 23155-859-25 50 in 1 BOTTLE; Type 0: Not a Combination Product 06/25/2023 11/30/2027 2 NDC: 23155-859-78 2 in 1 CARTON 06/25/2023 08/31/2027 2 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA065490 06/25/2023 11/30/2027 Labeler - Heritage Pharmaceuticals Inc. d/b/a Avet Pharmaceuticals Inc. (780779901) Establishment Name Address ID/FEI Business Operations Strides Pharma Science Limited 918513263 analysis(23155-858, 23155-859) , manufacture(23155-858, 23155-859) , pack(23155-858, 23155-859)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.