ENOXAPARIN SODIUM injection
Enoxaparin Sodium by
Drug Labeling and Warnings
Enoxaparin Sodium by is a Prescription medication manufactured, distributed, or labeled by Amphastar Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Enoxaparin Sodium Injection safely and effectively. See full prescribing information for Enoxaparin Sodium Injection.
Enoxaparin Sodium Injection for subcutaneous and intravenous use
Initial U.S. Approval: 1993WARNING: SPINAL/EPIDURAL HEMATOMA
See full prescribing information for complete boxed warning.
Epidural or spinal hematomas may occur in patients who are anticoagulated with low molecular weight heparins (LMWH) or heparinoids and are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling patients for spinal procedures. Factors that can increase the risk of developing epidural or spinal hematomas in these patients include:
- Use of indwelling epidural catheters
- Concomitant use of other drugs that affect hemostasis, such as non-steroidal anti-inflammatory drugs (NSAIDs), platelet inhibitors, other anticoagulants
- A history of traumatic or repeated epidural or spinal punctures
- A history of spinal deformity or spinal surgery
- Optimal timing between the administration of Enoxaparin Sodium Injection and neuraxial procedures is not known.
Monitor patients frequently for signs and symptoms of neurological impairment. If neurological compromise is noted, urgent treatment is necessary. [see Warnings and Precautions (5.1) and Drug Interactions (7)].
RECENT MAJOR CHANGES
Boxed Warning 11/13
Warnings and Precautions (5.1) 11/13INDICATIONS AND USAGE
Enoxaparin Sodium Injection is a low molecular weight heparin [LMWH] indicated for:
- Prophylaxis of deep vein thrombosis (DVT) in abdominal surgery, hip replacement surgery, knee replacement surgery, or medical patients with severely restricted mobility during acute illness (1.1)
- Inpatient treatment of acute DVT with or without pulmonary embolism (1.2)
- Outpatient treatment of acute DVT without pulmonary embolism (1.2)
- Prophylaxis of ischemic complications of unstable angina and non-Q-wave myocardial infarction [MI] (1.3)
- Treatment of acute ST-segment elevation myocardial infarction [STEMI] managed medically or with subsequent percutaneous coronary intervention [PCI] (1.4)
DOSAGE AND ADMINISTRATION
Indication Dose DVT prophylaxis in abdominal surgery 40 mg SC once daily DVT prophylaxis in knee replacement surgery 30 mg SC every 12 hours DVT prophylaxis in hip replacement surgery 30 mg SC every 12 hours or 40 mg SC once daily DVT prophylaxis in medical patients 40 mg SC once daily Inpatient treatment of acute DVT with or without pulmonary embolism 1 mg/kg SC every 12 hours or 1.5 mg/kg SC once daily* Outpatient treatment of acute DVT without pulmonary embolism 1 mg/kg SC every 12 hours* Unstable angina and non-Q-wave MI 1 mg/kg SC every 12 hours (with aspirin) Acute STEMI in patients <75 years of age [For dosing in subsequent PCI, see Dosage and Administration (2.1)] 30 mg single IV bolus plus a 1 mg/kg SC dose followed by 1 mg/kg SC every 12 hours (with aspirin) Acute STEMI in patients ≥75 years of age 0.75 mg/kg SC every 12 hours (no bolus) (with aspirin) DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Increased risk of hemorrhage: Use with caution in patients at risk (5.1)
- Percutaneous coronary revascularization: Obtain hemostasis at the puncture site before sheath removal (5.2)
- Concomitant medical conditions: Use with caution in patients with bleeding diathesis, uncontrolled arterial hypertension or history of recent gastrointestinal ulceration, diabetic retinopathy, renal dysfunction, or hemorrhage (5.3)
- History of heparin-induced thrombocytopenia: Use with caution (5.4)
- Thrombocytopenia: Monitor thrombocytopenia closely (5.5)
- Interchangeability with other heparins: Do not exchange with heparin or other LMWHs (5.6)
- Pregnant women with mechanical prosthetic heart valves and their fetuses, may be at increased risk and may need more frequent monitoring and dosage adjustment (5.7)
ADVERSE REACTIONS
Most common adverse reactions (>1%) were bleeding, anemia, thrombocytopenia, elevation of serum aminotransferase, diarrhea, and nausea. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Amphastar Pharmaceuticals, Inc. at 1-800-423-4136 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
- Severe Renal Impairment: Adjust dose for patients with creatinine clearance <30 mL/min (2.2, 8.7)
- Geriatric Patients: Monitor for increased risk of bleeding (8.5)
- Patients with mechanical heart valves: Not adequately studied (8.6)
- Hepatic Impairment: Use with caution (8.8)
- Low-Weight Patients: Observe for signs of bleeding (8.9)
- Obese Patients: Not adequately studied. Observe for thromboembolism (8.10)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 6/2017
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: SPINAL / EPIDURAL HEMATOMAS
1 INDICATIONS AND USAGE
1.1 Prophylaxis of Deep Vein Thrombosis
1.2 Treatment of Acute Deep Vein Thrombosis
1.3 Prophylaxis of Ischemic Complications of Unstable Angina and Non-Q-Wave Myocardial Infarction
1.4 Treatment of Acute ST-Segment Elevation Myocardial Infarction
2 DOSAGE AND ADMINISTRATION
2.1 Adult Dosage
2.2 Renal Impairment
2.3 Geriatric Patients with Acute ST-Segment Elevation Myocardial Infarction
2.4 Administration
3 DOSAGE FORMS AND STRENGTHS
3.1 100 mg/mL Concentration
3.2 150 mg/mL Concentration
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Increased Risk of Hemorrhage
5.2 Percutaneous Coronary Revascularization Procedures
5.3 Use of Enoxaparin Sodium Injection with Concomitant Medical Conditions
5.4 History of Heparin-Induced Thrombocytopenia
5.5 Thrombocytopenia
5.6 Interchangeability with Other Heparins
5.7 Pregnant Women with Mechanical Prosthetic Heart Valves
5.9 Laboratory Tests
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Patients with Mechanical Prosthetic Heart Valves
8.7 Renal Impairment
8.8 Hepatic Impairment
8.9 Low-Weight Patients
8.10 Obese Patients
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
13.3 Reproductive and Developmental Toxicology
14 CLINICAL STUDIES
14.1 Prophylaxis of Deep Vein Thrombosis Following Abdominal Surgery in Patients at Risk for Thromboembolic Complications
14.2 Prophylaxis of Deep Vein Thrombosis Following Hip or Knee Replacement Surgery
14.3 Prophylaxis of Deep Vein Thrombosis in Medical Patients with Severely Restricted Mobility During Acute Illness
14.4 Treatment of Deep Vein Thrombosis with or without Pulmonary Embolism
14.5 Prophylaxis of Ischemic Complications in Unstable Angina and Non-Q-Wave Myocardial Infarction
14.6 Treatment of Acute ST-Segment Elevation Myocardial Infarction
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: SPINAL / EPIDURAL HEMATOMAS
Epidural or spinal hematomas may occur in patients who are anticoagulated with low molecular weight heparins (LMWH) or heparinoids and are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling patients for spinal procedures. Factors that can increase the risk of developing epidural or spinal hematomas in these patients include:
- Use of indwelling epidural catheters
- Concomitant use of other drugs that affect hemostasis, such as non-steroidal anti-inflammatory drugs (NSAIDs), platelet inhibitors, other anticoagulants
- A history of traumatic or repeated epidural or spinal punctures
- A history of spinal deformity or spinal surgery
- Optimal timing between the administration of Enoxaparin Sodium Injection and neuraxial procedures is not known.
Monitor patients frequently for signs and symptoms of neurological impairment. If neurological compromise is noted, urgent treatment is necessary.
[see Warnings and Precautions (5.1) and Drug Interactions (7)].
-
1 INDICATIONS AND USAGE
1.1 Prophylaxis of Deep Vein Thrombosis
Enoxaparin Sodium Injection is indicated for the prophylaxis of deep vein thrombosis (DVT), which may lead to pulmonary embolism (PE):
- in patients undergoing abdominal surgery who are at risk for thromboembolic complications [see Clinical Studies 14.1].
- in patients undergoing hip replacement surgery, during and following hospitalization.
- in patients undergoing knee replacement surgery.
- in medical patients who are at risk for thromboembolic complications due to severely restricted mobility during acute illness.
1.2 Treatment of Acute Deep Vein Thrombosis
Enoxaparin Sodium Injection is indicated for:
- the inpatient treatment of acute deep vein thrombosis with or without pulmonary embolism, when administered in conjunction with warfarin sodium.
- the outpatient treatment of acute deep vein thrombosis without pulmonary embolism when administered in conjunction with warfarin sodium.
1.3 Prophylaxis of Ischemic Complications of Unstable Angina and Non-Q-Wave Myocardial Infarction
Enoxaparin Sodium Injection is indicated for the prophylaxis of ischemic complications of unstable angina and non-Q-wave myocardial infarction, when concurrently administered with aspirin.
1.4 Treatment of Acute ST-Segment Elevation Myocardial Infarction
Enoxaparin Sodium Injection, when administered concurrently with aspirin, has been shown to reduce the rate of the combined endpoint of recurrent myocardial infarction or death in patients with acute ST-segment elevation myocardial infarction (STEMI) receiving thrombolysis and being managed medically or with percutaneous coronary intervention (PCI).
-
2 DOSAGE AND ADMINISTRATION
All patients should be evaluated for a bleeding disorder before administration of Enoxaparin Sodium Injection, unless the medication is needed urgently. Since coagulation parameters are unsuitable for monitoring Enoxaparin Sodium Injection activity, routine monitoring of coagulation parameters is not required [see Warnings and Precautions (5.9)].
For subcutaneous use, Enoxaparin Sodium Injection should not be mixed with other injections or infusions.
For intravenous use (i.e., for treatment of acute STEMI), Enoxaparin Sodium Injection can be mixed with normal saline solution (0.9%) or 5% dextrose in water.
Enoxaparin Sodium Injection is not intended for intramuscular administration.
2.1 Adult Dosage
Abdominal Surgery: In patients undergoing abdominal surgery who are at risk for thromboembolic complications, the recommended dose of Enoxaparin Sodium Injection is 40 mg once a day administered by SC injection with the initial dose given 2 hours prior to surgery. The usual duration of administration is 7 to 10 days; up to 12 days administration has been administered in clinical trials.
Hip or Knee Replacement Surgery: In patients undergoing hip or knee replacement surgery, the recommended dose of Enoxaparin Sodium Injection is 30 mg every 12 hours administered by SC injection. Provided that hemostasis has been established, the initial dose should be given 12 to 24 hours after surgery. For hip replacement surgery, a dose of 40 mg once a day SC, given initially 12 (±3) hours prior to surgery, may be considered. Following the initial phase of thromboprophylaxis in hip replacement surgery patients, it is recommended that continued prophylaxis with Enoxaparin Sodium Injection 40 mg once a day be administered by SC injection for 3 weeks. The usual duration of administration is 7 to 10 days; up to 14 days administration has been administered in clinical trials.
Medical Patients During Acute Illness: In medical patients at risk for thromboembolic complications due to severely restricted mobility during acute illness, the recommended dose of Enoxaparin Sodium Injection is 40 mg once a day administered by SC injection. The usual duration of administration is 6 to 11 days; up to 14 days of Enoxaparin Sodium Injection has been administered in the controlled clinical trial.
Treatment of Deep Vein Thrombosis with or without Pulmonary Embolism: In outpatient treatment, patients with acute deep vein thrombosis without pulmonary embolism who can be treated at home, the recommended dose of Enoxaparin Sodium Injection is 1 mg/kg every 12 hours administered SC. In inpatient (hospital) treatment, patients with acute deep vein thrombosis with pulmonary embolism or patients with acute deep vein thrombosis without pulmonary embolism (who are not candidates for outpatient treatment), the recommended dose of Enoxaparin Sodium Injection is 1 mg/kg every 12 hours administered SC or 1.5 mg/kg once a day administered SC at the same time every day. In both outpatient and inpatient (hospital) treatments, warfarin sodium therapy should be initiated when appropriate (usually within 72 hours of Enoxaparin Sodium Injection). Enoxaparin Sodium Injection should be continued for a minimum of 5 days and until a therapeutic oral anticoagulant effect has been achieved (International Normalization Ratio 2.0 to 3.0). The average duration of administration is 7 days; up to 17 days of Enoxaparin Sodium Injection administration has been administered in controlled clinical trials.
Unstable Angina and Non-Q-Wave Myocardial Infarction: In patients with unstable angina or non-Q-wave myocardial infarction, the recommended dose of Enoxaparin Sodium Injection is 1 mg/kg administered SC every 12 hours in conjunction with oral aspirin therapy (100 to 325 mg once daily). Treatment with Enoxaparin Sodium Injection should be prescribed for a minimum of 2 days and continued until clinical stabilization. The usual duration of treatment is 2 to 8 days; up to 12.5 days of Enoxaparin Sodium Injection has been administered in clinical trials [see Warnings and Precautions (5.2) and Clinical Studies (14.5)].
Treatment of Acute ST-Segment Elevation Myocardial Infarction:
In patients with acute ST-segment elevation myocardial infarction, the recommended dose of Enoxaparin Sodium Injection is a single IV bolus of 30 mg plus a 1 mg/kg SC dose followed by 1 mg/kg administered SC every 12 hours (maximum 100 mg for the first two doses only, followed by 1 mg/kg dosing for the remaining doses). Dosage adjustments are recommended in patients ≥75 years of age [see Dosage and Administration (2.3)]. All patients should receive aspirin as soon as they are identified as having STEMI and maintained with 75 to 325 mg once daily unless contraindicated.
When administered in conjunction with a thrombolytic (fibrin-specific or non-fibrin specific), Enoxaparin Sodium Injection should be given between 15 minutes before and 30 minutes after the start of fibrinolytic therapy. In the pivotal clinical study, the Enoxaparin Sodium Injection treatment duration was 8 days or until hospital discharge, whichever came first. An optimal duration of treatment is not known, but it is likely to be longer than 8 days.
For patients managed with percutaneous coronary intervention (PCI): If the last Enoxaparin Sodium Injection SC administration was given less than 8 hours before balloon inflation, no additional dosing is needed. If the last Enoxaparin Sodium Injection SC administration was given more than 8 hours before balloon inflation, an IV bolus of 0.3 mg/kg of Enoxaparin Sodium Injection should be administered [see Warnings and Precautions (5.2)].
2.2 Renal Impairment
Although no dose adjustment is recommended in patients with moderate (creatinine clearance 30–50 mL/min) and mild (creatinine clearance 50–80 mL/min) renal impairment, all such patients should be observed carefully for signs and symptoms of bleeding.
The recommended prophylaxis and treatment dosage regimens for patients with severe renal impairment (creatinine clearance <30 mL/min) are described in Table 1 [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
Table 1 Dosage Regimens for Patients with Severe Renal Impairment
(creatinine clearance <30 mL/minute)Indication Dosage Regimen Prophylaxis in abdominal surgery 30 mg administered SC once daily Prophylaxis in hip or knee replacement surgery 30 mg administered SC once daily Prophylaxis in medical patients during acute illness 30 mg administered SC once daily Inpatient treatment of acute deep vein thrombosis with or without pulmonary embolism, when administered in conjunction with warfarin sodium 1 mg/kg administered SC once daily Outpatient treatment of acute deep vein thrombosis without pulmonary embolism, when administered in conjunction with warfarin sodium 1 mg/kg administered SC once daily Prophylaxis of ischemic complications of unstable angina and non-Q-wave myocardial infarction, when concurrently administered with aspirin 1 mg/kg administered SC once daily Treatment of acute ST-segment elevation myocardial infarction in patients <75 years of age, when administered in conjunction with aspirin 30 mg single IV bolus plus a 1 mg/kg SC dose followed by 1 mg/kg administered SC once daily Treatment of acute ST-segment elevation myocardial infarction in geriatric patients ≥75 years of age, when administered in conjunction with aspirin 1 mg/kg administered SC once daily (no initial bolus) 2.3 Geriatric Patients with Acute ST-Segment Elevation Myocardial Infarction
For treatment of acute ST-segment elevation myocardial infarction in geriatric patients ≥75 years of age, do not use an initial IV bolus. Initiate dosing with 0.75 mg/kg SC every 12 hours (maximum 75 mg for the first two doses only, followed by 0.75 mg/kg dosing for the remaining doses) [see Use in Specific Populations (8.5) and Clinical Phamacology (12.3)].
No dose adjustment is necessary for other indications in geriatric patients unless kidney function is impaired [see Dosage and Administration (2.2)].
2.4 Administration
Enoxaparin Sodium Injection is a clear, colorless to pale yellow sterile solution, and as with other parenteral drug products, should be inspected visually for particulate matter and discoloration prior to administration.
Enoxaparin Sodium Injection must not be administered by intramuscular injection.
Enoxaparin Sodium Injection is intended for use under the guidance of a physician.
For subcutaneous administration, patients may self-inject only if their physicians determine that it is appropriate and with medical follow-up, as necessary. Proper training in subcutaneous injection technique (with or without the assistance of an injection device) should be provided.
Subcutaneous Injection Technique: Patients should be lying down and Enoxaparin Sodium Injection administered by deep SC injection. To avoid the loss of drug when using the 30 and 40 mg prefilled syringes, do not expel the air bubble from the syringe before the injection. Administration should be alternated between the left and right anterolateral and left and right posterolateral abdominal wall. The whole length of the needle should be introduced into a skin fold held between the thumb and forefinger; the skin fold should be held throughout the injection. To minimize bruising, do not rub the injection site after completion of the injection.
Enoxaparin Sodium Injection prefilled syringes and graduated prefilled syringes are for single, one-time use only and are available with a system that shields the needle after injection.
Remove the prefilled syringe from the blister packaging by peeling at the arrow as directed on the blister. Do not remove by pulling on the plunger as this may damage the syringe.
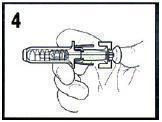
- Remove needle cover by pulling straight off of needle (see Figure 1). If adjusting the dose is required, the adjustment must be done prior to injecting the prescribed dose into the patient.
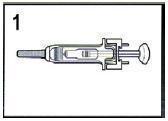
- See Administration: Subcutaneous Injection Technique for a description of the Standard Protocol for administration.
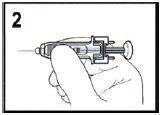
- Depress the plunger while grasping the finger flange until the entire dose has been given. The Passive needle guard should not activate unless the entire dose has been given.
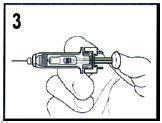
- Remove needle from patient, then let go of the plunger and allow syringe to move up until the entire needle is guarded.
- Dispose of syringe/needle guard assembly in approved sharps container.
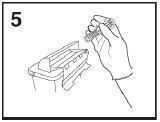
NOTE:
- The safety system should only activate once the syringe has been emptied.
- Activation of the safety system must be done only after removing the needle from the patient's skin.
- Do not replace the needle shield after injection.
- The safety system should not be sterilized.
Activation of the safety system may cause minimal splatter of fluid. For optimal safety activate the system while orienting it downwards away from yourself and others.
Intravenous (Bolus) Injection Technique
For intravenous injection, the multiple-dose vial should be used. Enoxaparin Sodium Injection should be administered through an intravenous line. Enoxaparin Sodium Injection should not be mixed or co-administered with other medications. To avoid the possible mixture of Enoxaparin Sodium Injection with other drugs, the intravenous access chosen should be flushed with a sufficient amount of saline or dextrose solution prior to and following the intravenous bolus administration of Enoxaparin Sodium Injection to clear the port of drug. Enoxaparin Sodium Injection may be safely administered with normal saline solution (0.9%) or 5% dextrose in water.
- Remove needle cover by pulling straight off of needle (see Figure 1). If adjusting the dose is required, the adjustment must be done prior to injecting the prescribed dose into the patient.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
- Active major bleeding
- Thrombocytopenia associated with a positive in vitro test for anti-platelet antibody in the presence of enoxaparin sodium
- Known hypersensitivity to enoxaparin sodium (e.g., pruritus, urticaria, anaphylactic/ anaphylactoid reactions) [see Adverse Reactions (6.2)]
- Known hypersensitivity to heparin or pork products
-
5 WARNINGS AND PRECAUTIONS
5.1 Increased Risk of Hemorrhage
Cases of epidural or spinal hemorrhage and subsequent hematomas have been reported with the use of Enoxaparin Sodium Injection and epidural or spinal anesthesia/analgesia or spinal puncture procedures, resulting in long-term or permanent paralysis. The risk of these events is higher with the use of post-operative indwelling epidural catheters, with the concomitant use of additional drugs affecting hemostasis such as NSAIDs, with traumatic or repeated epidural or spinal puncture, or in patients with a history of spinal surgery or spinal deformity [see Boxed Warning, Adverse Reactions (6.2) and Drug Interactions (7)].
To reduce the potential risk of bleeding associated with the concurrent use of enoxaparin sodium and epidural or spinal anesthesia/analgesia or spinal puncture, consider the pharmacokinetic profile of enoxaparin [see Clinical Pharmacology (12.3)]. Placement or removal of an epidural catheter or lumbar puncture is best performed when the anticoagulant effect of enoxaparin is low; however, the exact timing to reach a sufficiently low anticoagulant effect in each patient is not known.
Placement or removal of a catheter should be delayed for at least 12 hours after administration of lower doses (30 mg once or twice daily or 40 mg once daily) of Enoxaparin Sodium Injection, and at least 24 hours after the administration of higher doses (0.75 mg/kg twice daily, 1 mg/kg twice daily, or 1.5 mg/kg once daily) of Enoxaparin Sodium Injection. Anti-Xa levels are still detectable at these time points, and these delays are not a guarantee that neuraxial hematoma will be avoided. Patients receiving the 0.75 mg/kg twice daily dose or the 1 mg/kg twice daily dose should not receive the second enoxaparin dose in the twice daily regimen to allow a longer delay before catheter placement or removal. Likewise, although a specific recommendation for timing of a subsequent Enoxaparin Sodium Injection dose after catheter removal cannot be made, consider delaying this next dose for at least four hours, based on a benefit-risk assessment considering both the risk for thrombosis and the risk for bleeding in the context of the procedure and patient risk factors. For patients with creatinine clearance <30mL/minute, additional considerations are necessary because elimination of enoxaparin is more prolonged; consider doubling the timing of removal of a catheter, at least 24 hours for the lower prescribed dose of Enoxaparin Sodium Injection (30 mg once daily) and at least 48 hours for the higher dose (1 mg/kg/day) [see Clinical Pharmacology (12.3)].
Should the physician decide to administer anticoagulation in the context of epidural or spinal anesthesia/analgesia or lumbar puncture, frequent monitoring must be exercised to detect any signs and symptoms of neurological impairment such as midline back pain, sensory and motor deficits (numbness or weakness in lower limbs), bowel and/or bladder dysfunction. Instruct patients to report immediately if they experience any of the above signs or symptoms. If signs or symptoms of spinal hematoma are suspected, initiate urgent diagnosis and treatment including consideration for spinal cord decompression even though such treatment may not prevent or reverse neurological sequelae.
Enoxaparin sodium injection should be used with extreme caution in conditions with increased risk of hemorrhage, such as bacterial endocarditis, congenital or acquired bleeding disorders, active ulcerative and angiodysplastic gastrointestinal disease, hemorrhagic stroke, or shortly after brain, spinal, or ophthalmological surgery, or in patients treated concomitantly with platelet inhibitors.
Major hemorrhages including retroperitoneal and intracranial bleeding have been reported. Some of these cases have been fatal.
Bleeding can occur at any site during therapy with enoxaparin sodium injection. An unexplained fall in hematocrit or blood pressure should lead to a search for a bleeding site.
5.2 Percutaneous Coronary Revascularization Procedures
To minimize the risk of bleeding following the vascular instrumentation during the treatment of unstable angina, non-Q-wave myocardial infarction, and acute ST-segment elevation myocardial infarction, adhere precisely to the intervals recommended between enoxaparin sodium injection doses. It is important to achieve hemostasis at the puncture site after PCI.
In case a closure device is used, the sheath can be removed immediately. If a manual compression method is used, sheath should be removed 6 hours after the last IV/SC enoxaparin sodium injection. If the treatment with enoxaparin sodium is to be continued, the next scheduled dose should be given no sooner than 6 to 8 hours after sheath removal. The site of the procedure should be observed for signs of bleeding or hematoma formation [see Dosage and Administration (2.1)].
5.3 Use of Enoxaparin Sodium Injection with Concomitant Medical Conditions
Enoxaparin sodium injection should be used with care in patients with a bleeding diathesis, uncontrolled arterial hypertension or a history of recent gastrointestinal ulceration, diabetic retinopathy, renal dysfunction and hemorrhage.
5.4 History of Heparin-Induced Thrombocytopenia
Enoxaparin sodium injection should be used with extreme caution in patients with a history of heparin-induced thrombocytopenia.
5.5 Thrombocytopenia
Thrombocytopenia can occur with the administration of enoxaparin sodium injection.
Moderate thrombocytopenia (platelet counts between 100,000/mm3 and 50,000/mm3) occurred at a rate of 1.3% in patients given enoxaparin sodium injection, 1.2% in patients given heparin, and 0.7% in patients given placebo in clinical trials.
Platelet counts less than 50,000/mm3 occurred at a rate of 0.1% in patients given enoxaparin sodium injection, in 0.2% of patients given heparin, and 0.4% of patients given placebo in the same trials.
Thrombocytopenia of any degree should be monitored closely. If the platelet count falls below 100,000/mm3, enoxaparin sodium injection should be discontinued. Cases of heparin-induced thrombocytopenia with thrombosis have also been observed in clinical practice. Some of these cases were complicated by organ infarction, limb ischemia, or death [see Warnings and Precautions (5.4)].
5.6 Interchangeability with Other Heparins
Enoxaparin sodium injection cannot be used interchangeably (unit for unit) with heparin or other low molecular weight heparins as they differ in manufacturing process, molecular weight distribution, anti-Xa and anti-IIa activities, units, and dosage. Each of these medicines has its own instructions for use.
5.7 Pregnant Women with Mechanical Prosthetic Heart Valves
The use of enoxaparin sodium injection for thromboprophylaxis in pregnant women with mechanical prosthetic heart valves has not been adequately studied. In a clinical study of pregnant women with mechanical prosthetic heart valves given enoxaparin (1 mg/kg twice daily) to reduce the risk of thromboembolism, 2 of 8 women developed clots resulting in blockage of the valve and leading to maternal and fetal death. Although a causal relationship has not been established these deaths may have been due to therapeutic failure or inadequate anticoagulation. No patients in the heparin/warfarin group (0 of 4 women) died. There also have been isolated postmarketing reports of valve thrombosis in pregnant women with mechanical prosthetic heart valves while receiving enoxaparin for thromboprophylaxis. Women with mechanical prosthetic heart valves may be at higher risk for thromboembolism during pregnancy, and, when pregnant, have a higher rate of fetal loss from stillbirth, spontaneous abortion and premature delivery. Therefore, frequent monitoring of peak and trough anti-Factor Xa levels, and adjusting of dosage may be needed [see Use in Specific Populations (8.6)].
5.9 Laboratory Tests
Periodic complete blood counts, including platelet count, and stool occult blood tests are recommended during the course of treatment with enoxaparin sodium injection. When administered at recommended prophylaxis doses, routine coagulation tests such as Prothrombin Time (PT) and Activated Partial Thromboplastin Time (aPTT) are relatively insensitive measures of enoxaparin sodium injection activity and, therefore, unsuitable for monitoring. Anti-Factor Xa may be used to monitor the anticoagulant effect of enoxaparin sodium injection in patients with significant renal impairment. If during enoxaparin sodium injection therapy abnormal coagulation parameters or bleeding should occur, anti-Factor Xa levels may be used to monitor the anticoagulant effects of enoxaparin sodium injection [see Clinical Pharmacology (12.3)].
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
The following serious adverse reactions are also discussed in other sections of the labeling:
- Spinal/epidural hematoma [see Boxed Warning and Warnings and Precautions (5.1)]
- Increased Risk of Hemorrhage [see Warnings and Precautions (5.1)]
- Thrombocytopenia [see Warnings and Precautions (5.5)]
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
During clinical development for the approved indications, 15,918 patients were exposed to enoxaparin sodium. These included 1,228 for prophylaxis of deep vein thrombosis following abdominal surgery in patients at risk for thromboembolic complications, 1,368 for prophylaxis of deep vein thrombosis following hip or knee replacement surgery, 711 for prophylaxis of deep vein thrombosis in medical patients with severely restricted mobility during acute illness, 1,578 for prophylaxis of ischemic complications in unstable angina and non-Q-wave myocardial infarction, 10,176 for treatment of acute ST-elevation myocardial infarction, and 857 for treatment of deep vein thrombosis with or without pulmonary embolism. Enoxaparin sodium doses in the clinical trials for prophylaxis of deep vein thrombosis following abdominal or hip or knee replacement surgery or in medical patients with severely restricted mobility during acute illness ranged from 40 mg SC once daily to 30 mg SC twice daily. In the clinical studies for prophylaxis of ischemic complications of unstable angina and non-Q-wave myocardial infarction doses were 1 mg/kg every 12 hours and in the clinical studies for treatment of acute ST-segment elevation myocardial infarction enoxaparin sodium doses were a 30 mg IV bolus followed by 1 mg/kg every 12 hours SC.
Hemorrhage
The incidence of major hemorrhagic complications during enoxaparin sodium injection treatment has been low.
The following rates of major bleeding events have been reported during clinical trials with enoxaparin sodium injection [see Tables 2 to 7].
Table 2 Major Bleeding Episodes Following Abdominal and Colorectal Surgery* Indications Dosing Regimen Enoxaparin Sodium Inj. Heparin 40 mg q.d. SC 5000 U q8h SC - * Bleeding complications were considered major: (1) if the hemorrhage caused a significant clinical event, or (2) if accompanied by a hemoglobin decrease ≥2 g/dL or transfusion of 2 or more units of blood products. Retroperitoneal, intraocular, and intracranial hemorrhages were always considered major.
Abdominal Surgery n=555 n=560 23 (4%) 16 (3%) Colorectal Surgery n=673 n=674 28 (4%) 21 (3%) Table 3 Major Bleeding Episodes Following Hip or Knee Replacement Surgery* Dosing Regimen Indications Enoxaparin Sodium Inj.
40 mg q.d. SCEnoxaparin Sodium Inj.
30 mg q12h SCHeparin
15,000 U/24h SCNOTE: At no time point were the 40 mg once a day pre-operative and the 30 mg every 12 hours post-operative hip replacement surgery prophylactic regimens compared in clinical trials. Injection site hematomas during the extended prophylaxis period after hip replacement surgery occurred in 9% of the enoxaparin sodium injection patients versus 1.8% of the placebo patients. - * Bleeding complications were considered major: (1) if the hemorrhage caused a significant clinical event, or (2) if accompanied by a hemoglobin decrease ≥ 2 g/dL or transfusion of 2 or more units of blood products. Retroperitoneal and intracranial hemorrhages were always considered major. In the knee replacement surgery trials, intraocular hemorrhages were also considered major hemorrhages.
- † Enoxaparin sodium injection 30 mg every 12 hours SC initiated 12 to 24 hours after surgery and continued for up to 14 days after surgery.
- ‡ Enoxaparin sodium injection 40 mg SC once a day initiated up to 12 hours prior to surgery and continued for up to 7 days after surgery.
- § Enoxaparin sodium injection 40 mg SC once a day for up to 21 days after discharge.
Hip Replacement Surgery without Extended Prophylaxis† n = 786
31 (4%)n = 541
32 (6%)Hip Replacement Surgery with Extended Prophylaxis Peri-operative Period‡ n = 288 4 (2%) Extended Prophylaxis Period§ n = 221
0 (0%)Knee Replacement Surgery without Extended Prophylaxis† n = 294
3 (1%)n = 225
3 (1%)Table 4 Major Bleeding Episodes in Medical Patients with Severely Restricted Mobility During Acute Illness* Dosing Regimen Indications Enoxaparin Sodium Inj.† Enoxaparin Sodium Inj.† Placebo† 20 mg q.d. SC 40 mg q.d. SC - * Bleeding complications were considered major: (1) if the hemorrhage caused a significant clinical event, (2) if the hemorrhage caused a decrease in hemoglobin of ≥ 2 g/dL or transfusion of 2 or more units of blood products. Retroperitoneal and intracranial hemorrhages were always considered major although none were reported during the trial.
- † The rates represent major bleeding on study medication up to 24 hours after last dose.
Medical Patients During Acute Illness n = 351
1 (<1%)n = 360
3 (<1%)n = 362
2 (<1%)Table 5 Major Bleeding Episodes in Deep Vein Thrombosis with or without Pulmonary Embolism Treatment* Dosing Regimen† Indication Enoxaparin Sodium Inj.
1.5 mg/kg q.d. SCEnoxaparin Sodium Inj.
1 mg/kg q12h SCHeparin
aPTT Adjusted IV Therapy- * Bleeding complications were considered major: (1) if the hemorrhage caused a significant clinical event, or (2) if accompanied by a hemoglobin decrease ≥2 g/dL or transfusion of 2 or more units of blood products. Retroperitoneal, intraocular, and intracranial hemorrhages were always considered major.
- † All patients also received warfarin sodium (dose-adjusted according to PT to achieve an INR of 2.0 to 3.0) commencing within 72 hours of enoxaparin sodium injection or standard heparin therapy and continuing for up to 90 days.
Treatment of DVT and PE n = 298
5 (2%)n = 559
9 (2%)n = 554
9 (2%)Table 6 Major Bleeding Episodes in Unstable Angina and Non-Q-Wave Myocardial Infarction Dosing Regimen Indication Enoxaparin Sodium Inj.*
1 mg/kg q12h SCHeparin*
aPTT Adjusted IV Therapy- * The rates represent major bleeding on study medication up to 12 hours after dose.
- † Aspirin therapy was administered concurrently (100 to 325 mg per day).
- ‡ Bleeding complications were considered major: (1) if the hemorrhage caused a significant clinical event, or (2) if accompanied by a hemoglobin decrease by ≥ 3 g/dL or transfusion of 2 or more units of blood products. Intraocular, retroperitoneal, and intracranial hemorrhages were always considered major.
Unstable Angina and Non-Q-Wave MI †,‡ n = 1578
17 (1%)n = 1529
18 (1%)Table 7 Major Bleeding Episodes in Acute ST-Segment Elevation Myocardial Infarction Dosing Regimen Indication Enoxaparin Sodium Inj.*
Initial 30 mg IV bolus followed by 1 mg/kg q12h SCHeparin*
aPTT Adjusted IV Therapy- * The rates represent major bleeding (including ICH) up to 30 days
- † Bleedings were considered major if the hemorrhage caused a significant clinical event associated with a hemoglobin decrease by ≥ 5 g/dL. ICH were always considered major.
Acute ST-Segment Elevation n = 10176 n = 10151 Myocardial Infarction n (%) n (%) -Major bleeding (including ICH)† 211 (2.1) 138 (1.4) -Intracranial hemorrhages (ICH) 84 (0.8) 66 (0.7) Elevations of Serum Aminotransferases
Asymptomatic increases in aspartate (AST [SGOT]) and alanine (ALT [SGPT]) aminotransferase levels greater than three times the upper limit of normal of the laboratory reference range have been reported in up to 6.1% and 5.9% of patients, respectively, during treatment with enoxaparin sodium injection. Similar significant increases in aminotransferase levels have also been observed in patients and healthy volunteers treated with heparin and other low molecular weight heparins. Such elevations are fully reversible and are rarely associated with increases in bilirubin.
Since aminotransferase determinations are important in the differential diagnosis of myocardial infarction, liver disease, and pulmonary emboli, elevations that might be caused by drugs like enoxaparin sodium injection should be interpreted with caution.
Local Reactions
Mild local irritation, pain, hematoma, ecchymosis, and erythema may follow SC injection of enoxaparin sodium injection.
Adverse Reactions in Patients Receiving Enoxaparin Sodium Injection for Prophylaxis or Treatment of DVT, PE:
Other adverse reactions that were thought to be possibly or probably related to treatment with enoxaparin sodium injection, heparin, or placebo in clinical trials with patients undergoing hip or knee replacement surgery, abdominal or colorectal surgery, or treatment for DVT and that occurred at a rate of at least 2% in the enoxaparin sodium injection group, are provided below [see Tables 8 to 11].
Table 8 Adverse Reactions Occurring at ≥ 2% Incidence in Enoxaparin Sodium Injection-Treated Patients Undergoing Abdominal or Colorectal Surgery Dosing Regimen Enoxaparin Sodium Inj.
40 mg q.d. SC
n = 1228
%Heparin
5000 U q8h SC
n = 1234
%Adverse Reaction Severe Total Severe Total Hemorrhage <1 7 <1 6 Anemia <1 3 <1 3 Ecchymosis 0 3 0 3 Table 9 Adverse Reactions Occurring at ≥ 2% Incidence in Enoxaparin Sodium Injection-Treated Patients Undergoing Hip or Knee Replacement Surgery Dosing Regimen Enoxaparin Sodium Inj.
40 mg q.d. SCEnoxaparin
Sodium Inj.
30 mg q12h SCHeparin
15,000 U/24h SCPlacebo
q12h SCPeri-operative
PeriodExtended Prophylaxis
Periodn = 288*
%n = 131†
%n = 1080
%n = 766
%n = 115
%Adverse Reaction Severe Total Severe Total Severe Total Severe Total Severe Total - * Data represent enoxaparin sodium injection 40 mg SC once a day initiated up to 12 hours prior to surgery in 288 hip replacement surgery patients who received enoxaparin sodium injection peri-operatively in an unblinded fashion in one clinical trial.
- † Data represent enoxaparin sodium injection 40 mg SC once a day given in a blinded fashion as extended prophylaxis at the end of the peri-operative period in 131 of the original 288 hip replacement surgery patients for up to 21 days in one clinical trial.
Fever 0 8 0 0 <1 5 <1 4 0 3 Hemorrhage <1 13 0 5 <1 4 1 4 0 3 Nausea <1 3 <1 2 0 2 Anemia 0 16 0 <2 <1 2 2 5 <1 7 Edema <1 2 <1 2 0 2 Peripheral edema 0 6 0 0 <1 3 <1 4 0 3 Table 10 Adverse Reactions Occurring at ≥ 2% Incidence in Enoxaparin Sodium Injection-Treated Medical Patients with Severely Restricted Mobility During Acute Illness Adverse Reaction Dosing Regimen Enoxaparin Sodium Inj.
40 mg q.d. SC
n = 360
%Placebo
q.d. SC
n = 362
%Dyspnea 3.3 5.2 Thrombocytopenia 2.8 2.8 Confusion 2.2 1.1 Diarrhea 2.2 1.7 Nausea 2.5 1.7 Table 11 Adverse Reactions Occurring at ≥ 2% Incidence in Enoxaparin Sodium Injection-Treated Patients Undergoing Treatment of Deep Vein Thrombosis with or without Pulmonary Embolism Adverse Event Dosing Regimen Enoxaparin Sodium Inj.
1.5 mg/kg q.d. SC
n = 298
%Enoxaparin Sodium Inj.
1 mg/kg q12h SC
n = 559
%Heparin
aPTT Adjusted IV Therapy
n = 544
%Severe Total Severe Total Severe Total Injection Site Hemorrhage 0 5 0 3 <1 <1 Injection Site Pain 0 2 0 2 0 0 Hematuria 0 2 0 <1 <1 2 Adverse Events in Enoxaparin Sodium Injection-Treated Patients with Unstable Angina or Non-Q-Wave Myocardial Infarction:
Non-hemorrhagic clinical events reported to be related to enoxaparin sodium injection therapy occurred at an incidence of ≤ 1%.
Non-major hemorrhagic events, primarily injection site ecchymoses and hematomas, were more frequently reported in patients treated with SC enoxaparin sodium injection than in patients treated with IV heparin.
Serious adverse events with enoxaparin sodium injection or heparin in a clinical trial in patients with unstable angina or non-Q-wave myocardial infarction that occurred at a rate of at least 0.5% in the enoxaparin sodium injection group are provided below [see Table 12].
Table 12 Serious Adverse Events Occurring at ≥ 0.5% Incidence in Enoxaparin Sodium Injection-Treated Patients with Unstable Angina or Non-Q-Wave Myocardial Infarction Adverse Event Dosing Regimen Enoxaparin Sodium Inj.
1 mg/kg q12h SC
n =1578
n (%)Heparin
aPTT Adjusted IV Therapy
n =1529
n (%)Atrial fibrillation 11 (0.70) 3 (0.20) Heart failure 15 (0.95) 11 (0.72) Lung edema 11 (0.70) 11 (0.72) Pneumonia 13 (0.82) 9 (0.59) Adverse Reactions in Enoxaparin Sodium Injection-Treated Patients with Acute ST-Segment Elevation Myocardial Infarction:
In a clinical trial in patients with acute ST-segment elevation myocardial infarction, the only adverse reaction that occurred at a rate of at least 0.5% in the enoxaparin sodium injection group was thrombocytopenia (1.5%).
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of enoxaparin sodium injection. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
There have been reports of epidural or spinal hematoma formation with concurrent use of enoxaparin sodium injection and spinal/epidural anesthesia or spinal puncture. The majority of patients had a post-operative indwelling epidural catheter placed for analgesia or received additional drugs affecting hemostasis such as NSAIDs. Many of the epidural or spinal hematomas caused neurologic injury, including long-term or permanent paralysis.
Local reactions at the injection site (e.g., nodules, inflammation, oozing including shock), systemic allergic reactions (e.g., pruritus, urticaria, anaphylactic/anaphylactoid reactions), vesiculobullous rash, rare cases of hypersensitivity cutaneous vasculitis, purpura, skin necrosis (occurring at either the injection site or distant from the injection site), thrombocytosis, and thrombocytopenia with thrombosis [see Warnings and Precautions (5.5)] have been reported.
Cases of hyperkalemia have been reported. Most of these reports occurred in patients who also had conditions that tend toward the development of hyperkalemia (e.g., renal dysfunction, concomitant potassium-sparing drugs, administration of potassium, hematoma in body tissues). Very rare cases of hyperlipidemia have also been reported, with one case of hyperlipidemia, with marked hypertriglyceridemia, reported in a diabetic pregnant woman; causality has not been determined.
Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to estimate reliably their frequency or to establish a causal relationship to drug exposure.
Cases of headache, hemorrhagic anemia, eosinophilia, alopecia, hepatocellular and cholestatic liver injury have been reported. Osteoperosis has also been reported following long-term therapy.
-
7 DRUG INTERACTIONS
Whenever possible, agents which may enhance the risk of hemorrhage should be discontinued prior to initiation of enoxaparin sodium injection therapy. These agents include medications such as: anticoagulants, platelet inhibitors including acetylsalicylic acid, salicylates, NSAIDs (including ketorolac tromethamine), dipyridamole, or sulfinpyrazone. If co-administration is essential, conduct close clinical and laboratory monitoring [see Warnings and Precautions (5.9)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B
All pregnancies have a background risk of birth defect, loss, or other adverse outcome regardless of drug exposure. The fetal risk summary below describes the potential of enoxaparin sodium injection to increase the risk of developmental abnormalities above the background risk.
Fetal Risk Summary
Enoxaparin Sodium Injection does not cross the placenta, and is not expected to result in fetal exposure to the drug. Human data from a retrospective cohort study, which included 693 live births, suggest that Enoxaparin Sodium Injection does not increase the risk of major developmental abnormalities. Based on animal data, enoxaparin is not predicted to increase the risk of major developmental abnormalities (see Data).
Clinical Considerations
Pregnancy alone confers an increased risk for thromboembolism that is even higher for women with thromboembolic disease and certain high risk pregnancy conditions. While not adequately studied, pregnant women with mechanical prosthetic heart valves may be at even higher risk for thrombosis [see Warnings and Precautions (5.7) and Use in Specific Populations (8.6)]. Pregnant women with thromboembolic disease, including those with mechanical prosthetic heart valves and those with inherited or acquired thrombophilias, have an increased risk of other maternal complications and fetal loss regardless of the type of anticoagulant used.
All patients receiving anticoagulants, including pregnant women, are at risk for bleeding. Pregnant women receiving enoxaparin should be carefully monitored for evidence of bleeding or excessive anticoagulation. Consideration for use of a shorter acting anticoagulant should be specifically addressed as delivery approaches [see Boxed Warning]. Hemorrhage can occur at any site and may lead to death of mother and/or fetus. Pregnant women should be apprised of the potential hazard to the fetus and the mother if enoxaparin is administered during pregnancy.
It is not known if monitoring of anti-Factor Xa activity and dose adjustment (by weight or anti-Factor Xa activity) of Enoxaparin Sodium Injection affect the safety and the efficacy of the drug during pregnancy.
Data
-
Human Data - There are no adequate and well-controlled studies in pregnant women. A retrospective study reviewed the records of 604 women who used enoxaparin during pregnancy. A total of 624 pregnancies resulted in 693 live births. There were 72 hemorrhagic events (11 serious) in 63 women. There were 14 cases of neonatal hemorrhage. Major congenital anomalies in live births occurred at rates (2.5%) similar to background rates.
There have been postmarketing reports of fetal death when pregnant women received enoxaparin sodium injection. Causality for these cases has not been determined. Insufficient data, the underlying disease, and the possibility of inadequate anticoagulation complicate the evaluation of these cases.
A clinical study using enoxaparin in pregnant women with mechanical prosthetic heart valves has been conducted [see Warnings and Precautions (5.7)]. - Animal Data - Teratology studies have been conducted in pregnant rats and rabbits at SC doses of enoxaparin up to 15 times the recommended human dose (by comparison with 2 mg/kg as the maximum recommended daily dose). There was no evidence of teratogenic effects or fetotoxicity due to enoxaparin. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
8.3 Nursing Mothers
It is not known whether enoxaparin sodium injection is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from enoxaparin sodium injection, a decision should be made whether to discontinue nursing or discontinue enoxaparin sodium injection, taking into account the importance of enoxaparin sodium injection to the mother and the known benefits of nursing.
8.4 Pediatric Use
Safety and effectiveness of enoxaparin sodium injection in pediatric patients have not been established.
8.5 Geriatric Use
Prevention of Deep Vein Thrombosis in Hip, Knee and Abdominal Surgery; Treatment of Deep Vein Thrombosis, Prevention of Ischemic Complications of Unstable Angina and Non-Q-wave Myocardial Infarction
Over 2800 patients, 65 years and older, have received enoxaparin sodium injection in pivotal clinical trials. The efficacy of enoxaparin sodium injection in the geriatric (≥65 years) was similar to that seen in younger patients (<65 years). The incidence of bleeding complications was similar between geriatric and younger patients when 30 mg every 12 hours or 40 mg once a day doses of enoxaparin sodium injection were employed. The incidence of bleeding complications was higher in geriatric patients as compared to younger patients when enoxaparin sodium injection was administered at doses of 1.5 mg/kg once a day or 1 mg/kg every 12 hours. The risk of enoxaparin sodium injection-associated bleeding increased with age. Serious adverse events increased with age for patients receiving enoxaparin sodium injection. Other clinical experience (including postmarketing surveillance and literature reports) has not revealed additional differences in the safety of enoxaparin sodium injection between geriatric and younger patients. Careful attention to dosing intervals and concomitant medications (especially antiplatelet medications) is advised. Enoxaparin sodium injection should be used with care in geriatric patients who may show delayed elimination of enoxaparin. Monitoring of geriatric patients with low body weight (<45 kg) and those predisposed to decreased renal function should be considered [see Warnings and Precautions (5.9) and Clinical Pharmacology (12.3)].
Treatment of Acute ST-Segment Elevation Myocardial Infarction
In the clinical study for treatment of acute ST-segment elevation myocardial infarction, there was no evidence of difference in efficacy between patients ≥75 years of age (n = 1241) and patients less than 75 years of age (n = 9015). Patients ≥75 years of age did not receive a 30 mg IV bolus prior to the normal dosage regimen and had their SC dose adjusted to 0.75 mg/kg every 12 hours [see Dosage and Administration (2.3)]. The incidence of bleeding complications was higher in patients ≥65 years of age as compared to younger patients (<65 years).
8.6 Patients with Mechanical Prosthetic Heart Valves
The use of enoxaparin sodium injection has not been adequately studied for thromboprophylaxis in patients with mechanical prosthetic heart valves and has not been adequately studied for long-term use in this patient population. Isolated cases of prosthetic heart valve thrombosis have been reported in patients with mechanical prosthetic heart valves who have received enoxaparin for thromboprophylaxis. Some of these cases were pregnant women in whom thrombosis led to maternal and fetal deaths. Insufficient data, the underlying disease and the possibility of inadequate anticoagulation complicate the evaluation of these cases. Pregnant women with mechanical prosthetic heart valves may be at higher risk for thromboembolism [see Warnings and Precautions (5.7)].
8.7 Renal Impairment
In patients with renal impairment, there is an increase in exposure of enoxaparin sodium. All such patients should be observed carefully for signs and symptoms of bleeding. Because exposure of enoxaparin sodium is significantly increased in patients with severe renal impairment (creatinine clearance <30 mL/min), a dosage adjustment is recommended for therapeutic and prophylactic dosage ranges. No dosage adjustment is recommended in patients with moderate (creatinine clearance 30–50 mL/min) and mild (creatinine clearance 50–80 mL/min) renal impairment [see Dosage and Administration (2.2) and Clinical Pharmacology (12.3)]. In patients with renal failure, treatment with enoxaparin has been associated with the development of hyperkalemia [see Adverse Reactions (6.2)].
8.8 Hepatic Impairment
The impact of hepatic impairment on enoxaparin's exposure and antithrombotic effect has not been investigated. Caution should be exercised when administering enoxaparin to patients with hepatic impairment.
8.9 Low-Weight Patients
An increase in exposure of enoxaparin sodium with prophylactic dosages (non-weight adjusted) has been observed in low-weight women (<45 kg) and low-weight men (<57 kg). All such patients should be observed carefully for signs and symptoms of bleeding [see Clinical Pharmacology (12.3)].
8.10 Obese Patients
Obese patients are at higher risk for thromboembolism. The safety and efficacy of prophylactic doses of Enoxaparin Sodium Injection in obese patients (BMI >30 kg/m2) has not been fully determined and there is no consensus for dose adjustment. These patients should be observed carefully for signs and symptoms of thromboembolism.
-
Human Data - There are no adequate and well-controlled studies in pregnant women. A retrospective study reviewed the records of 604 women who used enoxaparin during pregnancy. A total of 624 pregnancies resulted in 693 live births. There were 72 hemorrhagic events (11 serious) in 63 women. There were 14 cases of neonatal hemorrhage. Major congenital anomalies in live births occurred at rates (2.5%) similar to background rates.
-
10 OVERDOSAGE
Accidental overdosage following administration of enoxaparin sodium injection may lead to hemorrhagic complications. Injected enoxaparin sodium injection may be largely neutralized by the slow IV injection of protamine sulfate (1% solution). The dose of protamine sulfate should be equal to the dose of enoxaparin sodium injection injected: 1 mg protamine sulfate should be administered to neutralize 1 mg enoxaparin sodium injection, if enoxaparin sodium was administered in the previous 8 hours. An infusion of 0.5 mg protamine per 1 mg of enoxaparin sodium may be administered if enoxaparin sodium was administered greater than 8 hours previous to the protamine administration, or if it has been determined that a second dose of protamine is required. The second infusion of 0.5 mg protamine sulfate per 1 mg of enoxaparin sodium injection may be administered if the aPTT measured 2 to 4 hours after the first infusion remains prolonged.
If at least 12 hours have elapsed since the last enoxaparin sodium injection, protamine administration may not be required; however, even with higher doses of protamine, the aPTT may remain more prolonged than following administration of heparin. In all cases, the anti-Factor Xa activity is never completely neutralized (maximum about 60%). Particular care should be taken to avoid overdosage with protamine sulfate. Administration of protamine sulfate can cause severe hypotensive and anaphylactoid reactions. Because fatal reactions, often resembling anaphylaxis, have been reported with protamine sulfate, it should be given only when resuscitation techniques and treatment of anaphylactic shock are readily available. For additional information consult the labeling of protamine sulfate injection products.
-
11 DESCRIPTION
Enoxaparin Sodium Injection is a sterile aqueous solution containing enoxaparin sodium, a low molecular weight heparin. The pH of the injection is 5.5 to 7.5.
Enoxaparin sodium is obtained by alkaline depolymerization of heparin benzyl ester derived from porcine intestinal mucosa. Its structure is characterized by a 2-O-sulfo-4-enepyranosuronic acid group at the non-reducing end and a 2-N,6-O-disulfo-D-glucosamine at the reducing end of the chain. About 20% (ranging between 15% and 25%) of the enoxaparin structure contains an 1,6 anhydro derivative on the reducing end of the polysaccharide chain. The drug substance is the sodium salt. The average molecular weight is about 4500 daltons. The molecular weight distribution is:
<2000 daltons ≤20% 2000 to 8000 daltons ≥68% >8000 daltons ≤18% - * X = Percent of polysaccharide chain containing 1,6 anhydro derivative on the reducing end.
STRUCTURAL FORMULA 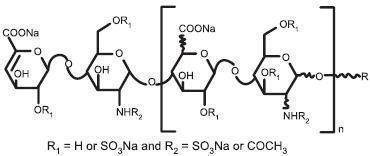
R X*=15 to 25% 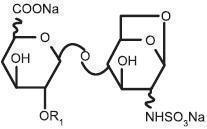
n=0 to 20 100 - X H n=1 to 21 Enoxaparin Sodium Injection 100 mg/mL Concentration contains 10 mg enoxaparin sodium (approximate anti-Factor Xa activity of 1000 IU [with reference to the W.H.O. Second International Low Molecular Weight Heparin Reference Standard]) per 0.1 mL Water for Injection.
Enoxaparin Sodium Injection 150 mg/mL Concentration contains 15 mg enoxaparin sodium (approximate anti-Factor Xa activity of 1500 IU [with reference to the W.H.O. Second International Low Molecular Weight Heparin Reference Standard]) per 0.1 mL Water for Injection.
The Enoxaparin Sodium Injection prefilled syringes and graduated prefilled syringes are preservative-free and intended for use only as a single-dose injection [see Dosage and Administration (2) and How Supplied (16)].
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Enoxaparin is a low molecular weight heparin, which has antithrombotic properties.
12.2 Pharmacodynamics
In humans, enoxaparin given at a dose of 1.5 mg/kg subcutaneously (SC) is characterized by a higher ratio of anti-Factor Xa to anti-Factor IIa activity (mean±SD, 14.0±3.1) (based on areas under anti-Factor activity versus time curves) compared to the ratios observed for heparin (mean±SD, 1.22±0.13). Increases of up to 1.8 times the control values were seen in the thrombin time (TT) and the activated partial thromboplastin time (aPTT). Enoxaparin at a 1 mg/kg dose (100 mg/mL concentration), administered SC every 12 hours to patients in a large clinical trial resulted in aPTT values of 45 seconds or less in the majority of patients (n =1607). A 30 mg IV bolus immediately followed by a 1 mg/kg SC administration resulted in aPTT post-injection values of 50 seconds. The average aPTT prolongation value on Day 1 was about 16% higher than on Day 4.
12.3 Pharmacokinetics
Absorption: Pharmacokinetic trials were conducted using the 100 mg/mL formulation. Maximum anti-Factor Xa and anti-thrombin (anti-Factor IIa) activities occur 3 to 5 hours after SC injection of enoxaparin. Mean peak anti-Factor Xa activity was 0.16 IU/mL (1.58 mcg/mL) and 0.38 IU/mL (3.83 mcg/mL) after the 20 mg and the 40 mg clinically tested SC doses, respectively. Mean (n = 46) peak anti-Factor Xa activity was 1.1 IU/mL at steady state in patients with unstable angina receiving 1 mg/kg SC every 12 hours for 14 days. Mean absolute bioavailability of enoxaparin, after 1.5 mg/kg given SC, based on anti-Factor Xa activity is approximately 100% in healthy subjects.
A 30 mg IV bolus immediately followed by a 1 mg/kg SC every 12 hours provided initial peak anti-Factor Xa levels of 1.16 IU/mL (n=16) and average exposure corresponding to 84% of steady-state levels. Steady state is achieved on the second day of treatment.
Enoxaparin pharmacokinetics appear to be linear over the recommended dosage ranges [see Dosage and Administration (2)]. After repeated subcutaneous administration of 40 mg once daily and 1.5 mg/kg once-daily regimens in healthy volunteers, the steady state is reached on day 2 with an average exposure ratio about 15% higher than after a single dose. Steady-state enoxaparin activity levels are well predicted by single-dose pharmacokinetics. After repeated subcutaneous administration of the 1 mg/kg twice daily regimen, the steady state is reached from day 4 with mean exposure about 65% higher than after a single dose and mean peak and trough levels of about 1.2 and 0.52 IU/mL, respectively. Based on enoxaparin sodium pharmacokinetics, this difference in steady state is expected and within the therapeutic range.
Although not studied clinically, the 150 mg/mL concentration of enoxaparin sodium is projected to result in anticoagulant activities similar to those of 100 mg/mL and 200 mg/mL concentrations at the same enoxaparin dose. When a daily 1.5 mg/kg SC injection of enoxaparin sodium was given to 25 healthy male and female subjects using a 100 mg/mL or a 200 mg/mL concentration the following pharmacokinetic profiles were obtained [see Table 13].
Table 13 Pharmacokinetic Parameters* After 5 Days of 1.5 mg/kg SC Once Daily Doses of Enoxaparin Sodium Using 100 mg/mL or 200 mg/mL Concentrations Concentration Anti-Xa Anti-IIa Heptest aPTT - * Means ± SD at Day 5 and 90% Confidence Interval (CI) of the ratio
- † Median (range)
Amax
(IU/mL or Δ sec)100 mg/mL 1.37 (±0.23) 0.23 (±0.05) 105 (±17) 19 (±5) 200 mg/mL 1.45 (±0.22) 0.26 (±0.05) 111 (±17) 22 (±7) 90% CI 102–110% 102–111% tmax†(h) 100 mg/mL 3 (2–6) 4 (2–5) 2.5 (2–4.5) 3 (2–4.5) 200 mg/mL 3.5 (2–6) 4.5 (2.5–6) 3.3 (2–5) 3 (2–5) AUC (ss)
(h*IU/mL or h*Δ sec)100 mg/mL 14.26 (±2.93) 1.54 (±0.61) 1321 (±219) 200 mg/mL 15.43 (±2.96) 1.77 (±0.67) 1401 (±227) 90% CI 105–112% 103–109% Elimination: Following intravenous (IV) dosing, the total body clearance of enoxaparin is 26 mL/min. After IV dosing of enoxaparin labeled with the gamma-emitter, 99mTc, 40% of radioactivity and 8 to 20% of anti-Factor Xa activity were recovered in urine in 24 hours. Elimination half-life based on anti-Factor Xa activity was 4.5 hours after a single SC dose to about 7 hours after repeated dosing. Significant anti-Factor Xa activity persists in plasma for about 12 hours following a 40 mg SC once a day dose.
Following SC dosing, the apparent clearance (CL/F) of enoxaparin is approximately 15 mL/min.
Metabolism: Enoxaparin sodium is primarily metabolized in the liver by desulfation and/or depolymerization to lower molecular weight species with much reduced biological potency. Renal clearance of active fragments represents about 10% of the administered dose and total renal excretion of active and non-active fragments 40% of the dose.
Special Populations
Gender: Apparent clearance and Amax derived from anti-Factor Xa values following single SC dosing (40 mg and 60 mg) were slightly higher in males than in females. The source of the gender difference in these parameters has not been conclusively identified; however, body weight may be a contributing factor.
Geriatric: Apparent clearance and Amax derived from anti-Factor Xa values following single and multiple SC dosing in geriatric subjects were close to those observed in young subjects. Following once a day SC dosing of 40 mg enoxaparin, the Day 10 mean area under anti-Factor Xa activity versus time curve (AUC) was approximately 15% greater than the mean Day 1 AUC value [see Dosage and Administration (2.3) and Use in Specific Populations (8.5)].
Renal Impairment: A linear relationship between anti-Factor Xa plasma clearance and creatinine clearance at steady-state has been observed, which indicates decreased clearance of enoxaparin sodium in patients with reduced renal function. Anti-Factor Xa exposure represented by AUC, at steady-state, is marginally increased in mild (creatinine clearance 50–80 mL/min) and moderate (creatinine clearance 30–50 mL/min) renal impairment after repeated subcutaneous 40 mg once-daily doses. In patients with severe renal impairment (creatinine clearance <30 mL/min), the AUC at steady-state is significantly increased on average by 65% after repeated subcutaneous 40 mg once-daily doses [see Dosage and Administration (2.2) and Use in Specific Populations (8.7)].
Hemodialysis: In a single study, elimination rate appeared similar but AUC was two-fold higher than control population, after a single 0.25 or 0.5 mg/kg intravenous dose.
Hepatic Impairment: Studies with enoxaparin in patients with hepatic impairment have not been conducted and the impact of hepatic impairment on the exposure to enoxaparin is unknown [see Use in Specific Populations (8.8)].
Weight: After repeated subcutaneous 1.5 mg/kg once daily dosing, mean AUC of anti-Factor Xa activity is marginally higher at steady-state in obese healthy volunteers (BMI 30–48 kg/m2) compared to non-obese control subjects, while Amax is not increased. When non-weight adjusted dosing was administered, it was found after a single-subcutaneous 40 mg dose, that anti-Factor Xa exposure is 52% higher in low-weight women (<45 kg) and 27% higher in low-weight men (< 57 kg) when compared to normal weight control subjects [see Use in Specific Populations (8.9)].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term studies in animals have been performed to evaluate the carcinogenic potential of enoxaparin. Enoxaparin was not mutagenic in in vitro tests, including the Ames test, mouse lymphoma cell forward mutation test, and human lymphocyte chromosomal aberration test, and the in vivo rat bone marrow chromosomal aberration test. Enoxaparin was found to have no effect on fertility or reproductive performance of male and female rats at SC doses up to 20 mg/kg/day or 141 mg/m2/day. The maximum human dose in clinical trials was 2.0 mg/kg/day or 78 mg/m2/day (for an average body weight of 70 kg, height of 170 cm, and body surface area of 1.8 m2).
13.2 Animal Toxicology and/or Pharmacology
A single SC dose of 46.4 mg/kg enoxaparin was lethal to rats. The symptoms of acute toxicity were ataxia, decreased motility, dyspnea, cyanosis, and coma.
13.3 Reproductive and Developmental Toxicology
Teratology studies have been conducted in pregnant rats and rabbits at SC doses of enoxaparin up to 30 mg/kg/day corresponding to 211 mg/m2/day and 410 mg/m2/day in rats and rabbits respectively. There was no evidence of teratogenic effects or fetotoxicity due to enoxaparin.
-
14 CLINICAL STUDIES
14.1 Prophylaxis of Deep Vein Thrombosis Following Abdominal Surgery in Patients at Risk for Thromboembolic Complications
Abdominal surgery patients at risk include those who are over 40 years of age, obese, undergoing surgery under general anesthesia lasting longer than 30 minutes or who have additional risk factors such as malignancy or a history of deep vein thrombosis (DVT) or pulmonary embolism (PE).
In a double-blind, parallel group study of patients undergoing elective cancer surgery of the gastrointestinal, urological, or gynecological tract, a total of 1116 patients were enrolled in the study, and 1115 patients were treated. Patients ranged in age from 32 to 97 years (mean age 67 years) with 52.7% men and 47.3% women. Patients were 98% Caucasian, 1.1% Black, 0.4% Asian, and 0.4% others. Enoxaparin sodium injection 40 mg SC, administered once a day, beginning 2 hours prior to surgery and continuing for a maximum of 12 days after surgery, was comparable to heparin 5000 U every 8 hours SC in reducing the risk of DVT. The efficacy data are provided below [see Table 14].
Table 14 Efficacy of Enoxaparin Sodium Injection in the Prophylaxis of Deep Vein Thrombosis Following Abdominal Surgery Indication Dosing Regimen Enoxaparin Sodium Inj.
40 mg q.d. SC
n (%)Heparin
5000 U q8h SC
n (%)- * VTE = Venous thromboembolic events which included DVT, PE, and death considered to be thromboembolic in origin.
- † CI = Confidence Interval
All Treated Abdominal Surgery Patients 555 (100) 560 (100) Treatment Failures Total VTE* (%) 56 (10.1)
(95% CI†: 8 to 13)63 (11.3)
(95% CI: 9 to 14)DVT Only (%) 54 (9.7)
(95% CI: 7 to 12)61 (10.9)
(95% CI: 8 to 13)In a second double-blind, parallel group study, enoxaparin sodium injection 40 mg SC once a day was compared to heparin 5000 U every 8 hours SC in patients undergoing colorectal surgery (one-third with cancer). A total of 1347 patients were randomized in the study and all patients were treated. Patients ranged in age from 18 to 92 years (mean age 50.1 years) with 54.2% men and 45.8% women. Treatment was initiated approximately 2 hours prior to surgery and continued for approximately 7 to 10 days after surgery. The efficacy data are provided below [see Table 15].
Table 15 Efficacy of Enoxaparin Sodium Injection in the Prophylaxis of Deep Vein Thrombosis Following Colorectal Surgery Dosing Regimen Enoxaparin Sodium Inj. Heparin Indication 40 mg q.d. SC
n (%)5000 U q8h SC
n (%)- * VTE = Venous thromboembolic events which included DVT, PE, and death considered to be thromboembolic in origin.
- † CI = Confidence Interval
All Treated Colorectal Surgery Patients 673 (100) 674 (100) Treatment Failures
Total VTE* (%)48 (7.1)
(95% CI† : 5 to 9)45 (6.7)
(95% CI: 5 to 9)DVT Only (%) 47 (7.0)
(95% CI: 5 to 9)44 (6.5)
(95% CI: 5 to 8)14.2 Prophylaxis of Deep Vein Thrombosis Following Hip or Knee Replacement Surgery
Enoxaparin sodium injection has been shown to reduce the risk of post-operative deep vein thrombosis (DVT) following hip or knee replacement surgery.
In a double-blind study, enoxaparin sodium injection 30 mg every 12 hours SC was compared to placebo in patients with hip replacement. A total of 100 patients were randomized in the study and all patients were treated. Patients ranged in age from 41 to 84 years (mean age 67.1 years) with 45% men and 55% women. After hemostasis was established, treatment was initiated 12 to 24 hours after surgery and was continued for 10 to 14 days after surgery. The efficacy data are provided below [see Table 16].
Table 16 Efficacy of Enoxaparin Sodium Injection in the Prophylaxis of Deep Vein Thrombosis Following Hip Replacement Surgery Indication Dosing Regimen Enoxaparin Sodium Inj.
30 mg q12h SC
n (%)Placebo
q12h SC
n (%)- * p value versus placebo = 0.0002
- † p value versus placebo = 0.0002
All Treated Hip Replacement Patients 50 (100) 50 (100) Treatment Failures Total DVT (%) 5 (10)* 23 (46) Proximal DVT (%) 1 (2)† 11 (22) A double-blind, multicenter study compared three dosing regimens of enoxaparin sodium injection in patients with hip replacement. A total of 572 patients were randomized in the study and 568 patients were treated. Patients ranged in age from 31 to 88 years (mean age 64.7 years) with 63% men and 37% women. Patients were 93% Caucasian, 6% Black, < 1% Asian, and 1% others. Treatment was initiated within two days after surgery and was continued for 7 to 11 days after surgery. The efficacy data are provided below [see Table 17].
Table 17 Efficacy of Enoxaparin Sodium Injection in the Prophylaxis of Deep Vein Thrombosis Following Hip Replacement Surgery Indication Dosing Regimen 10 mg q.d. SC
n (%)30 mg q12h SC
n (%)40 mg q.d. SC
n (%)- * p value versus placebo = 0.0002
- † p value versus placebo = 0.0002
All Treated Hip Replacement Patients 161 (100) 208 (100) 199 (100) Treatment Failures Total DVT (%) 40 (25) 22 (11)* 27 (14) Proximal DVT (%) 17 (11) 8 (4)† 9 (5) There was no significant difference between the 30 mg every 12 hours and 40 mg once a day regimens. In a double-blind study, enoxaparin sodium injection 30 mg every 12 hours SC was compared to placebo in patients undergoing knee replacement surgery. A total of 132 patients were randomized in the study and 131 patients were treated, of which 99 had total knee replacement and 32 had either unicompartmental knee replacement or tibial osteotomy. The 99 patients with total knee replacement ranged in age from 42 to 85 years (mean age 70.2 years) with 36.4% men and 63.6% women. After hemostasis was established, treatment was initiated 12 to 24 hours after surgery and was continued up to 15 days after surgery. The incidence of proximal and total DVT after surgery was significantly lower for enoxaparin sodium injection compared to placebo. The efficacy data are provided below [see Table 18].
Table 18 Efficacy of Enoxaparin Sodium Injection in the Prophylaxis of Deep Vein Thrombosis Following Total Knee Replacement Surgery Indication Dosing Regimen Enoxaparin Sodium Inj.
30 mg q12h SC
n (%)Placebo
q12h SC
n (%)- * p value versus placebo = 0.0002
- † p value versus placebo = 0.0002
- ‡ p value versus placebo = 0.0002
- § p value versus placebo = 0.0002
All Treated Total Knee Replacement Patients 47 (100) 52 (100) Treatment Failures 5 (11)* 32 (62) Total DVT (%) (95% CI†: 1 to 21) (95% CI: 47 to 76) Proximal DVT (%) 0 (0)‡
(95% Upper CL§: 5)7 (13)
(95% CI: 3 to 24)Additionally, in an open-label, parallel group, randomized clinical study, enoxaparin sodium injection 30 mg every 12 hours SC in patients undergoing elective knee replacement surgery was compared to heparin 5000 U every 8 hours SC. A total of 453 patients were randomized in the study and all were treated. Patients ranged in age from 38 to 90 years (mean age 68.5 years) with 43.7% men and 56.3% women. Patients were 92.5% Caucasian, 5.3% Black, and 0.6% others. Treatment was initiated after surgery and continued up to 14 days. The incidence of deep vein thrombosis was significantly lower for enoxaparin sodium injection compared to heparin.
Extended Prophylaxis of Deep Vein Thrombosis Following Hip Replacement Surgery: In a study of extended prophylaxis for patients undergoing hip replacement surgery, patients were treated, while hospitalized, with enoxaparin sodium injection 40 mg SC, initiated up to 12 hours prior to surgery for the prophylaxis of post-operative DVT. At the end of the peri-operative period, all patients underwent bilateral venography. In a double-blind design, those patients with no venous thromboembolic disease were randomized to a post-discharge regimen of either enoxaparin sodium injection 40 mg (n = 90) once a day SC or to placebo (n = 89) for 3 weeks. A total of 179 patients were randomized in the double-blind phase of the study and all patients were treated. Patients ranged in age from 47 to 87 years (mean age 69.4 years) with 57% men and 43% women. In this population of patients, the incidence of DVT during extended prophylaxis was significantly lower for enoxaparin sodium injection compared to placebo. The efficacy data are provided below [see Table 19].
Table 19 Efficacy of Enoxaparin Sodium Injection in the Extended Prophylaxis of Deep Vein Thrombosis Following Hip Replacement Surgery Indication (Post-Discharge) Post-Discharge Dosing Regimen Enoxaparin Sodium Inj.
40 mg q.d. SC
n (%)Placebo
q.d. SC
n (%)- * p value versus placebo = 0.0002
- † p value versus placebo = 0.0002
- ‡ p value versus placebo = 0.0002
All Treated Extended Prophylaxis Patients 90 (100) 89 (100) Treatment Failures
Total DVT (%)6 (7)*
(95% CI†: 3 to 14)18 (20)
(95% CI: 12 to 30)Proximal DVT (%) 5 (6)‡
(95% CI: 2 to 13)7 (8)
(95% CI: 3 to 16)In a second study, patients undergoing hip replacement surgery were treated, while hospitalized, with enoxaparin sodium injection 40 mg SC, initiated up to 12 hours prior to surgery. All patients were examined for clinical signs and symptoms of venous thromboembolic (VTE) disease. In a double-blind design, patients without clinical signs and symptoms of VTE disease were randomized to a post-discharge regimen of either enoxaparin sodium injection 40 mg (n = 131) once a day SC or to placebo (n = 131) for 3 weeks. A total of 262 patients were randomized in the study double-blind phase and all patients were treated. Patients ranged in age from 44 to 87 years (mean age 68.5 years) with 43.1% men and 56.9% women. Similar to the first study the incidence of DVT during extended prophylaxis was significantly lower for enoxaparin sodium injection compared to placebo, with a statistically significant difference in both total DVT (enoxaparin sodium injection 21 [16%] versus placebo 45 [34%]; p = 0.001) and proximal DVT (enoxaparin sodium injection 8 [6%] versus placebo 28 [21%]; p = <0.001).
14.3 Prophylaxis of Deep Vein Thrombosis in Medical Patients with Severely Restricted Mobility During Acute Illness
In a double blind multicenter, parallel group study, enoxaparin sodium injection 20 mg or 40 mg once a day SC was compared to placebo in the prophylaxis of deep vein thrombosis (DVT) in medical patients with severely restricted mobility during acute illness (defined as walking distance of <10 meters for ≤3 days). This study included patients with heart failure (NYHA Class III or IV); acute respiratory failure or complicated chronic respiratory insufficiency (not requiring ventilatory support): acute infection (excluding septic shock); or acute rheumatic disorder [acute lumbar or sciatic pain, vertebral compression (due to osteoporosis or tumor), acute arthritic episodes of the lower extremities]. A total of 1102 patients were enrolled in the study, and 1073 patients were treated. Patients ranged in age from 40 to 97 years (mean age 73 years) with equal proportions of men and women. Treatment continued for a maximum of 14 days (median duration 7 days). When given at a dose of 40 mg once a day SC, enoxaparin sodium injection significantly reduced the incidence of DVT as compared to placebo. The efficacy data are provided below [see Table 20].
Table 20 Efficacy of Enoxaparin Sodium Injection in the Prophylaxis of Deep Vein Thrombosis in Medical Patients with Severely Restricted Mobility During Acute Illness Indication Dosing Regimen Enoxaparin Sodium Inj.
20 mg q.d. SCEnoxaparin Sodium Inj.
40 mg q.d. SCPlacebo n (%) n (%) n (%) - * Treatment failures during therapy, between Days 1 and 14.
- † VTE = Venous thromboembolic events which included DVT, PE, and death considered to be thromboembolic in origin.
- ‡ CI = Confidence Interval
All Treated Medical Patients During Acute Illness 351 (100) 360 (100) 362 (100) Treatment Failure*
Total VTE† (%)43 (12.3) 16 (4.4) 43 (11.9) Total DVT (%) 43 (12.3)
(95% CI‡ 8.8 to 15.7)16 (4.4)
(95% CI‡ 2.3 to 6.6)41 (11.3)
(95% CI‡ 8.1 to 14.6)Proximal DVT (%) 13 (3.7) 5 (1.4) 14 (3.9) At approximately 3 months following enrollment, the incidence of venous thromboembolism remained significantly lower in the enoxaparin sodium injection 40 mg treatment group versus the placebo treatment group.
14.4 Treatment of Deep Vein Thrombosis with or without Pulmonary Embolism
In a multicenter, parallel group study, 900 patients with acute lower extremity deep vein thrombosis (DVT) with or without pulmonary embolism (PE) were randomized to an inpatient (hospital) treatment of either (i) enoxaparin sodium injection 1.5 mg/kg once a day SC, (ii) enoxaparin sodium injection 1 mg/kg every 12 hours SC, or (iii) heparin IV bolus (5000 IU) followed by a continuous infusion (administered to achieve an aPTT of 55 to 85 seconds). A total of 900 patients were randomized in the study and all patients were treated. Patients ranged in age from 18 to 92 years (mean age 60.7 years) with 54.7% men and 45.3% women. All patients also received warfarin sodium (dose adjusted according to PT to achieve an International Normalization Ratio [INR] of 2.0 to 3.0), commencing within 72 hours of initiation of enoxaparin sodium injection or standard heparin therapy, and continuing for 90 days. Enoxaparin sodium injection or standard heparin therapy was administered for a minimum of 5 days and until the targeted warfarin sodium INR was achieved. Both enoxaparin sodium injection regimens were equivalent to standard heparin therapy in reducing the risk of recurrent venous thromboembolism (DVT and/or PE). The efficacy data are provided below [see Table 21].
Table 21 Efficacy of Enoxaparin Sodium Injection in Treatment of Deep Vein Thrombosis with or without Pulmonary Embolism Indication Dosing Regimen* Enoxaparin Sodium Inj.
1.5 mg/kg q.d. SC
n (%)Enoxaparin Sodium Inj.
1 mg/kg q12h SC
n (%)Heparin
aPTT Adjusted
IV Therapy
n (%)- * All patients were also treated with warfarin sodium commencing within 72 hours of enoxaparin sodium injection or standard heparin therapy.
- † VTE = venous thromboembolic event (DVT and/or PE).
- ‡ The 95% Confidence Intervals for the treatment differences for total VTE were: enoxaparin sodium injection once a day versus heparin (-3.0 to 3.5) enoxaparin sodium injection every 12 hours versus heparin (-4.2 to 1.7).
All Treated DVT Patients with or without PE 298 (100) 312 (100) 290 (100) Patient Outcome
Total VTE† (%)13 (4.4)‡ 9 (2.9)‡ 12 (4.1) DVT Only (%) 11 (3.7) 7 (2.2) 8 (2.8) Proximal DVT (%) 9 (3.0) 6 (1.9) 7 (2.4) PE (%) 2 (0.7) 2 (0.6) 4 (1.4) Similarly, in a multicenter, open-label, parallel group study, patients with acute proximal DVT were randomized to enoxaparin sodium injection or heparin. Patients who could not receive outpatient therapy were excluded from entering the study. Outpatient exclusion criteria included the following: inability to receive outpatient heparin therapy because of associated co-morbid conditions or potential for non-compliance and inability to attend follow-up visits as an outpatient because of geographic inaccessibility. Eligible patients could be treated in the hospital, but ONLY enoxaparin sodium injection patients were permitted to go home on therapy (72%). A total of 501 patients were randomized in the study and all patients were treated. Patients ranged in age from 19 to 96 years (mean age 57.8 years) with 60.5% men and 39.5% women. Patients were randomized to either enoxaparin sodium injection 1 mg/kg every 12 hours SC or heparin IV bolus (5000 IU) followed by a continuous infusion administered to achieve an aPTT of 60 to 85 seconds (in-patient treatment). All patients also received warfarin sodium as described in the previous study. Enoxaparin sodium injection or standard heparin therapy was administered for a minimum of 5 days. Enoxaparin sodium injection was equivalent to standard heparin therapy in reducing the risk of recurrent venous thromboembolism. The efficacy data are provided below [see Table 22].
Table 22 Efficacy of Enoxaparin Sodium Injection in Treatment of Deep Vein Thrombosis Indication Dosing Regimen* Enoxaparin Sodium Inj.
1 mg/kg q12h SC
n (%)Heparin
aPTT Adjusted
IV Therapy
n (%)- * All patients were also treated with warfarin sodium commencing on the evening of the second day of enoxaparin sodium injection or standard heparin therapy.
- † VTE = venous thromboembolic event (deep vein thrombosis [DVT] and/or pulmonary embolism [PE]).
- ‡ The 95% Confidence Intervals for the treatment difference for total VTE was: enoxaparin sodium injection versus heparin (-5.6 to 2.7).
All Treated DVT Patients 247 (100) 254 (100) Patient Outcome
Total VTE† (%)13 (5.3)‡ 17 (6.7) DVT Only (%) 11 (4.5) 14 (5.5) Proximal DVT (%) 10 (4.0) 12 (4.7) PE (%) 2 (0.8) 3 (1.2) 14.5 Prophylaxis of Ischemic Complications in Unstable Angina and Non-Q-Wave Myocardial Infarction
In a multicenter, double-blind, parallel group study, patients who recently experienced unstable angina or non-Q-wave myocardial infarction were randomized to either enoxaparin sodium injection 1 mg/kg every 12 hours SC or heparin IV bolus (5000 U) followed by a continuous infusion (adjusted to achieve an aPTT of 55 to 85 seconds). A total of 3171 patients were enrolled in the study, and 3107 patients were treated. Patients ranged in age from 25–94 years (median age 64 years), with 33.4% of patients female and 66.6% male. Race was distributed as follows: 89.8% Caucasian, 4.8% Black, 2.0% Asian, and 3.5% other. All patients were also treated with aspirin 100 to 325 mg per day. Treatment was initiated within 24 hours of the event and continued until clinical stabilization, revascularization procedures, or hospital discharge, with a maximal duration of 8 days of therapy. The combined incidence of the triple endpoint of death, myocardial infarction, or recurrent angina was lower for enoxaparin sodium injection compared with heparin therapy at 14 days after initiation of treatment. The lower incidence of the triple endpoint was sustained up to 30 days after initiation of treatment. These results were observed in an analysis of both all-randomized and all-treated patients. The efficacy data are provided below [see Table 23].
Table 23 Efficacy of Enoxaparin Sodium Injection in the Prophylaxis of Ischemic Complications in Unstable Angina and Non-Q-Wave Myocardial Infarction (Combined Endpoint of Death, Myocardial Infarction, or Recurrent Angina) Indication Dosing Regimen* Enoxaparin Sodium Inj.
1 mg/kg q12h SC
n (%)Heparin
aPTT Adjusted
IV Therapy
n (%)Reduction
(%)p Value - * All patients were also treated with aspirin 100 to 325 mg per day.
- † Evaluation timepoints are after initiation of treatment. Therapy continued for up to 8 days (median duration of 2.6 days).
All Treated Unstable Angina and Non-Q-Wave MI Patients 1578 (100) 1529 (100) Timepoint † 48 Hours 96 (6.1) 112 (7.3) 1.2 0.120 14 Days 261 (16.5) 303 (19.8) 3.3 0.017 30 Days 313 (19.8) 358 (23.4) 3.6 0.014 The combined incidence of death or myocardial infarction at all time points was lower for enoxaparin sodium injection compared to standard heparin therapy, but did not achieve statistical significance. The efficacy data are provided below [see Table 24].
Table 24 Efficacy of Enoxaparin Sodium Injection in the Prophylaxis of Ischemic Complications in Unstable Angina and Non-Q-Wave Myocardial Infarction (Combined Endpoint of Death or Myocardial Infarction) Dosing Regimen* Indication Enoxaparin Sodium Inj.
1 mg/kg q12h SC
n (%)Heparin
aPTT Adjusted
IV Therapy
n (%)Reduction
(%)p Value - * All patients were also treated with aspirin 100 to 325 mg per day.
- † Evaluation timepoints are after initiation of treatment. Therapy continued for up to 8 days (median duration of 2.6 days).
All Treated Unstable Angina and Non-Q-Wave MI Patients 1578 (100) 1529 (100) Timepoint † 48 Hours 16 (1.0) 20 (1.3) 0.3 0.126 14 Days 76 (4.8) 93 (6.1) 1.3 0.115 30 Days 96 (6.1) 118 (7.7) 1.6 0.069 In a survey one year following treatment, with information available for 92% of enrolled patients, the combined incidence of death, myocardial infarction, or recurrent angina remained lower for enoxaparin sodium injection versus heparin (32.0% vs 35.7%).
Urgent revascularization procedures were performed less frequently in the enoxaparin sodium injection group as compared to the heparin group, 6.3% compared to 8.2% at 30 days (p = 0.047).
14.6 Treatment of Acute ST-Segment Elevation Myocardial Infarction
In a multicenter, double-blind, double-dummy, parallel group study, patients with acute ST-segment elevation myocardial infarction (STEMI) who were to be hospitalized within 6 hours of onset and were eligible to receive fibrinolytic therapy were randomized in a 1:1 ratio to receive either enoxaparin sodium injection or unfractionated heparin.
Study medication was initiated between 15 minutes before and 30 minutes after the initiation of fibrinolytic therapy. Unfractionated heparin was administered beginning with an IV bolus of 60 U/kg (maximum 4000 U) and followed with an infusion of 12 U/kg per hour (initial maximum 1000 U per hour) that was adjusted to maintain an aPTT of 1.5 to 2 times the control value. The IV infusion was to be given for at least 48 hours. The enoxaparin dosing strategy was adjusted according to the patient's age and renal function. For patients younger than 75 years of age, enoxaparin was given as a single 30 mg intravenous bolus plus a 1 mg/kg SC dose followed by an SC injection of 1 mg/kg every 12 hours. For patients at least 75 years of age, the IV bolus was not given and the SC dose was reduced to 0.75 mg/kg every 12 hours. For patients with severe renal insufficiency (estimated creatinine clearance of less than 30 mL per minute), the dose was to be modified to 1 mg/kg every 24 hours. The SC injections of enoxaparin were given until hospital discharge or for a maximum of eight days (whichever came first). The mean treatment duration for enoxaparin was 6.6 days. The mean treatment duration of unfractionated heparin was 54 hours.
When percutaneous coronary intervention was performed during study medication period, patients received antithrombotic support with blinded study drug. For patients on enoxaparin, the PCI was to be performed on enoxaparin (no switch) using the regimen established in previous studies, i.e. no additional dosing, if the last SC administration was less than 8 hours before balloon inflation, IV bolus of 0.3 mg/kg enoxaparin if the last SC administration was more than 8 hours before balloon inflation.
All patients were treated with aspirin for a minimum of 30 days. Eighty percent of patients received a fibrin-specific agent (19% tenecteplase, 5% reteplase and 55% alteplase) and 20% received streptokinase.
Among 20,479 patients in the ITT population, the mean age was 60 years, and 76% were male. Racial distribution was: 87% Caucasian, 9.8% Asian, 0.2% Black, and 2.8% other. Medical history included previous MI (13%), hypertension (44%), diabetes (15%) and angiographic evidence of CAD (5%). Concomitant medication included aspirin (95%), beta- blockers (86%), ACE inhibitors (78%), statins (70%) and clopidogrel (27%). The MI at entry was anterior in 43%, non-anterior in 56%, and both in 1%.
The primary efficacy end point was the composite of death from any cause or myocardial re-infarction in the first 30 days after randomization. Total follow-up was one year.
The rate of the primary efficacy end point (death or myocardial re-infarction) was 9.9% in the enoxaparin group, and 12.0% in the unfractionated heparin group, a 17% reduction in the relative risk, (P=0.000003) [see Table 25].
Table 25 Efficacy of Enoxaparin Sodium Injection in the Treatment of Acute ST-Segment Elevation Myocardial Infarction Enoxaparin
(N=10,256)UFH
(N=10,223)Relative Risk
(95% CI)P Value Note: Urgent revascularization denotes episodes of recurrent myocardial ischemia (without infarction) leading to the clinical decision to perform coronary revascularization during the same hospitalization. CI denotes confidence intervals. Outcome at 48 hours n (%) n (%) Death or Myocardial Re-infarction 478 (4.7) 531 (5.2) 0.90 (0.80 to 1.01) 0.08 Death 383 (3.7) 390 (3.8) 0.98 (0.85 to 1.12) 0.76 Myocardial Re-infarction 102 (1.0) 156 (1.5) 0.65 (0.51 to 0.84) <0.001 Urgent Revascularization 74 (0.7) 96 (0.9) 0.77 (0.57 to 1.04) 0.09 Death or Myocardial Re-infarction or Urgent Revascularization 548 (5.3) 622 (6.1) 0.88 (0.79 to 0.98) 0.02 Outcome at 8 Days Death or Myocardial Re-infarction 740 (7.2) 954 (9.3) 0.77 (0.71 to 0.85) <0.001 Death 559 (5.5) 605 (5.9) 0.92 (0.82 to 1.03) 0.15 Myocardial Re-infarction 204 (2.0) 379 (3.7) 0.54 (0.45 to 0.63) <0.001 Urgent Revascularization 145 (1.4) 247 (2.4) 0.59 (0.48 to 0.72) <0.001 Death or Myocardial Re-infarction or Urgent Revascularization 874 (8.5) 1181 (11.6) 0.74 (0.68 to 0.80) <0.001 Outcome at 30 Days Primary efficacy endpoint
(Death or Myocardial Re-infarction)1017 (9.9) 1223 (12.0) 0.83 (0.77 to 0.90) 0.000003 Death 708 (6.9) 765 (7.5) 0.92 (0.84 to 1.02) 0.11 Myocardial Re-infarction 352 (3.4) 508 (5.0) 0.69 (0.60 to 0.79) <0.001 Urgent Revascularization 213 (2.1) 286 (2.8) 0.74 (0.62 to 0.88) <0.001 Death or Myocardial Re-infarction or Urgent Revascularization 1199 (11.7) 1479 (14.5) 0.81 (0.75 to 0.87) <0.001 The beneficial effect of enoxaparin on the primary end point was consistent across key subgroups including age, gender, infarct location, history of diabetes, history of prior myocardial infarction, fibrinolytic agent administered, and time to treatment with study drug (see Figure 1); however, it is necessary to interpret such subgroup analyses with caution.
Figure 1.
Relative Risks of and Absolute Event Rates for the Primary End Point
at 30 Days in Various Subgroups *- * The primary efficacy end point was the composite of death from any cause or myocardial re-infarction in the first 30 days. The overall treatment effect of enoxaparin as compared to the unfractionated heparin is shown at the bottom of the figure. For each subgroup, the circle is proportional to the number and represents the point estimate of the treatment effect and the horizontal lines represent the 95 percent confidence intervals. Fibrin-specific fibrinolytic agents included alteplase, tenecteplase and reteplase. Time to treatment indicates the time from the onset of symptoms to the administration of study drug (median, 3.2 hours).
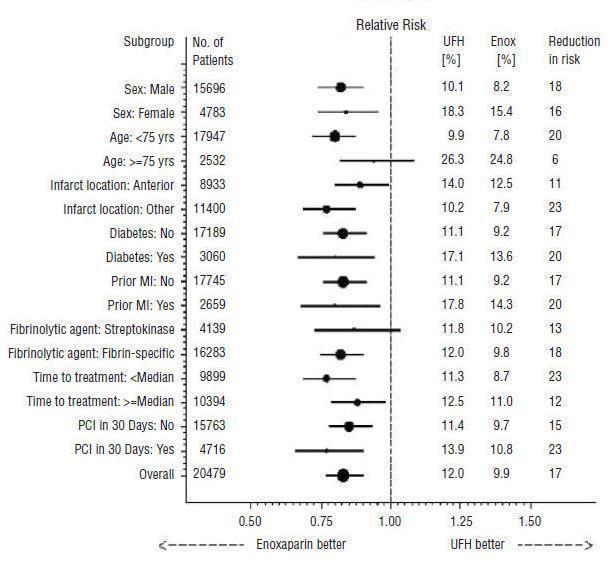
The beneficial effect of enoxaparin on the primary end point observed during the first 30 days was maintained over a 12 month follow-up period (see Figure 2).
Figure 2.
Kaplan-Meier plot - death or myocardial re-infarction at 30 days - ITT population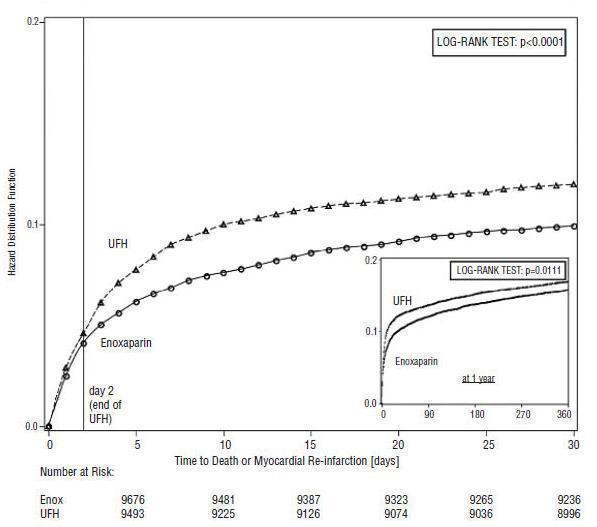
There is a trend in favor of enoxaparin during the first 48 hours, but most of the treatment difference is attributed to a step increase in the event rate in the UFH group at 48 hours (seen in Figure 2), an effect that is more striking when comparing the event rates just prior to and just subsequent to actual times of discontinuation. These results provide evidence that UFH was effective and that it would be better if used longer than 48 hours. There is a similar increase in endpoint event rate when enoxaparin was discontinued, suggesting that it too was discontinued too soon in this study.
The rates of major hemorrhages (defined as requiring 5 or more units of blood for transfusion, or 15% drop in hematocrit or clinically overt bleeding, including intracranial hemorrhage) at 30 days were 2.1% in the enoxaparin group and 1.4% in the unfractionated heparin group. The rates of intracranial hemorrhage at 30 days were 0.8% in the enoxaparin group 0.7% in the unfractionated heparin group. The 30-day rate of the composite endpoint of death, myocardial re-infarction or ICH (a measure of net clinical benefit) was significantly lower in the enoxaparin group (10.1%) as compared to the heparin group (12.2%).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Enoxaparin Sodium Injection is available in two concentrations [see Tables 26 and 27]:
Table 26 100 mg/mL Concentration Dosage Unit/Strength * Anti-Xa Activity † Package Size
(per carton)Syringe Label Color NDC# 0548- - * Strength represents the number of milligrams of enoxaparin sodium in Water for Injection. Enoxaparin Sodium Injection, 30 and 40 mg prefilled syringes, and 60, 80, and 100 mg graduated prefilled syringes each contain
- † Strength represents the number of milligrams of enoxaparin sodium in Water for Injection. Enoxaparin Sodium Injection, 30 and 40 mg prefilled syringes, and 60, 80, and 100 mg graduated prefilled syringes each contain
- ‡ Each Enoxaparin Sodium Injection prefilled syringe is for single, one-time use only and is affixed with a 27 gauge × 1/2 inch needle.
Prefilled Syringes‡
30 mg/0.3 mL3000 IU 10 syringes Medium
Blue5631-00 40 mg/0.4 mL 4000 IU 10 syringes Yellow 5632-00 Graduated Prefilled Syringes‡ 60 mg/0.6 mL 6000 IU 10 syringes Orange 5633-00 80 mg/0.8 mL 8000 IU 10 syringes Brown 5634-00 100 mg/1 mL 10,000 IU 10 syringes Black 5635-00 Table 27 150 mg/mL Concentration Dosage Unit /Strength * Anti-Xa Activity † Package Size
(per carton)Syringe Label Color NDC # 0548- - * Strength represents the number of milligrams of enoxaparin sodium in Water for Injection. Enoxaparin Sodium Injection, 30 and 40 mg prefilled syringes, and 60, 80, and 100 mg graduated prefilled syringes each contain
- † Strength represents the number of milligrams of enoxaparin sodium in Water for Injection. Enoxaparin Sodium Injection, 30 and 40 mg prefilled syringes, and 60, 80, and 100 mg graduated prefilled syringes each contain
- ‡ Strength represents the number of milligrams of enoxaparin sodium in Water for Injection. Enoxaparin Sodium Injection, 30 and 40 mg prefilled syringes, and 60, 80, and 100 mg graduated prefilled syringes each contain
Graduated Prefilled Syringes ‡ 120 mg/0.8 mL 12,000 IU 10 syringes Purple 5636-00 150 mg/1 mL 15,000 IU 10 syringes Navy Blue 5637-00 -
17 PATIENT COUNSELING INFORMATION
If patients have had neuraxial anesthesia or spinal puncture, and particularly, if they are taking concomitant NSAIDs, platelet inhibitors, or other anticoagulants, they should be informed to watch for signs and symptoms of spinal or epidural hematoma, such as tingling, numbness (especially in the lower limbs) and muscular weakness. If any of these symptoms occur the patient should contact his or her physician immediately.
Additionally, the use of aspirin and other NSAID's may enhance the risk of hemorrhage. Their use should be discontinued prior to enoxaparin therapy whenever possible; if co-administration is essential, the patient's clinical and laboratory status should be closely monitored [see Drug Interactions (7)].
Patients should also be informed:
- of the instructions for injecting Enoxaparin Sodium Injection if their therapy is to continue after discharge from the hospitals.
- it may take them longer than usual to stop bleeding.
- they may bruise and/or bleed more easily when they are treated with Enoxaparin Sodium Injection.
- they should report any unusual bleeding, bruising, or signs of thrombocytopenia (such as a rash of dark red spots under the skin) to their physician [see Warnings and Precautions (5.1, 5.5)].
- to tell their physicians and dentists they are taking Enoxaparin Sodium Injection and/or any other product known to affect bleeding before any surgery is scheduled and before any new drug is taken [see Warnings and Precautions (5.3)].
- to tell their physicians and dentists of all medications they are taking, including those obtained without a prescription, such as aspirin or other NSAID's [see Drug Interactions (7)].
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL: 10 x 30 mg Syringe Carton
ENOXAPARIN SODIUM INJECTION
30 mg/0.3 mL
NDC: 0548-5631-00
Rx ONLY
FOR SUBCUTANEOUS INJECTION
10 x 30 mg Single Dose Syringes with
Automatic Safety Device
Manufactured for Amerinet Choice by
AMPHASTAR PHARMACEUTICALS, INC.
Rancho Cucamonga, CA 91730, U.S.A.
Made in U.S.A.
© 2012

-
PRINCIPAL DISPLAY PANEL: 30 mg Syringe Peel Lid
Remove syringe by peeling lid completely off. Then gently lift syringe straight up by grasping middle of the syringe. Do not remove syringe by pulling on the plunger. See www.amphastar.com
ENOXAPARIN SODIUM INJECTION
30 mg/0.3 mL
Each 0.3 mL contains 30 mg of enoxaparin sodium derived from porcine intestinal mucosa in Water for Injection. See insert for directions for use.
FOR SUBCUTANEOUS INJECTION
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [See USP Controlled Room Temperature]
RX ONLY
WARNING: KEEP OUT OF REACH OF CHILDREN
Manufactured for Amerinet Choice by
AMPHASTAR PHARMACEUTICALS, INC.
Rancho Cucamonga, CA 91730, U.S.A.
Made in U.S.A.
8856316C 11-12
Stock No. 5631
NDC: 0548-5631-00
1 Single Dose Prefilled Syringe with Automatic Safety Device
One 0.3 mL Syringe
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.

-
PRINCIPAL DISPLAY PANEL: 10 x 40 mg Syringe Carton
ENOXAPARIN SODIUM INJECTION
40 mg/0.4 mL
NDC: 0548-5632-00
Rx ONLY
FOR SUBCUTANEOUS INJECTION
10 x 40 mg Single Dose Syringes with Automatic Safety Device
Manufactured for Amerinet Choice by
AMPHASTAR PHARMACEUTICALS, INC.
Rancho Cucamonga, CA 91730, U.S.A.
Made in U.S.A.
© 2012

-
PRINCIPAL DISPLAY PANEL: 40 mg Syringle Peel Lid
Remove syringe by peeling lid completely off. Then gently lift syringe straight up by grasping middle of the syringe. Do not remove syringe by pulling on the plunger. See www.amphastar.com
ENOXAPARIN SODIUM INJECTION
40 mg/0.4 mL
Each 0.4 mL contains 40 mg of enoxaparin sodium derived from porcine intestinal mucosa in Water for Injection. See insert for directions for use.
FOR SUBCUTANEOUS INJECTION
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [See USP Controlled Room Temperature]
RX ONLY
WARNING: KEEP OUT OF REACH OF CHILDREN
Manufactured for Amerinet Choice by
AMPHASTAR PHARMACEUTICALS, INC.
Rancho Cucamonga, CA 91730, U.S.A.
Made in U.S.A.
8856326C 11-12
Stock No. 5632
NDC: 0548-5632-00
1 Single Dose Prefilled Syringe with Automatic Safety Device
One 0.4 mL Syringe
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
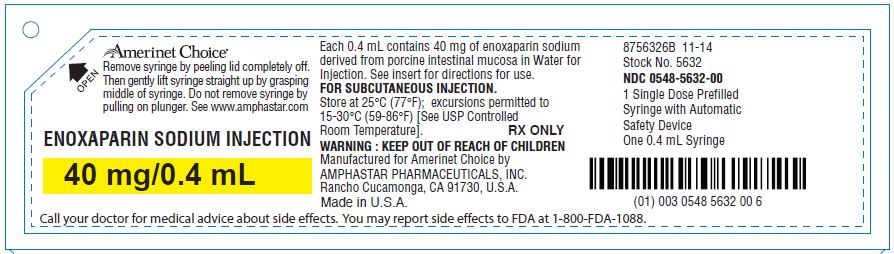
-
PRINCIPAL DISPLAY PANEL: 10 x 60 mg Syringe Carton
ENOXAPARIN SODIUM INJECTION
60 mg/0.6 mL
NDC: 0548-5633-00
Rx ONLY
FOR SUBCUTANEOUS INJECTION
10 x 60 mg Single Dose Syringes with Automatic Safety Device
Manufactured for Amerinet Choice by
AMPHASTAR PHARMACEUTICALS, INC.
Rancho Cucamonga, CA 91730, U.S.A.
Made in U.S.A.
© 2012
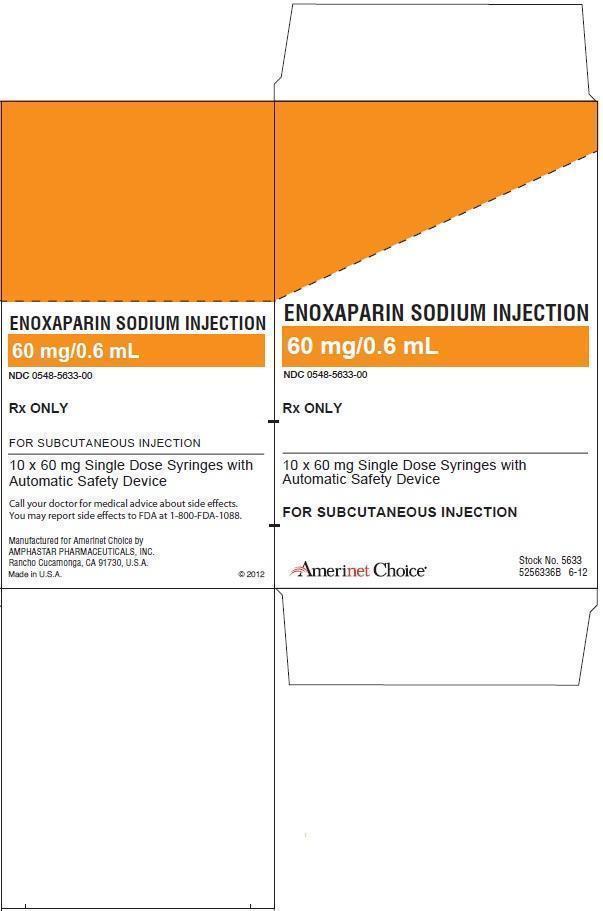

-
PRINCIPAL DISPLAY PANEL: 60 mg Syringe Peel Lid
Manufactured for Amerinet Choice by
AMPHASTAR PHARMACEUTICALS, INC.
Rancho Cucamonga, CA 91730, U.S.A.
Remove syringe by peeling lid completely off. Then gently lift straight up by grasping middle of syringe. Do not remove syringe by pulling on plunger. See www.amphastar.com
ENOXAPARIN SODIUM INJECTION
60 mg/0.6 mL
Each 0.6 mL contains 60 mg of enoxaparin sodium derived from porcine mucosa in Water for Injection. See insert for directions for use.
FOR SUBCUTANEOUS INJECTION
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [See USP Controlled Room Temperature].
WARNING: KEEP OUT OF REACH OF CHILDREN
Each 0.025 mL graduation equals 2.5 mg Enoxaparin Sodium Injection
RX ONLY
8856336C 11-12
Stock No. 5633
NDC: 0548-5633-00
1 Single Dose Prefilled Syringe with Automatic Safety Device
One 0.6 mL Syringe
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.

-
PRINCIPAL DISPLAY PANEL: 10 x 80 mg Syringe Carton
ENOXAPARIN SODIUM INJECTION
80 mg/0.8 mL
NDC: 0548-5634-00
Rx ONLY
FOR SUBCUTANEOUS INJECTION
10 x 80 mg Single Dose Syringes with Automatic Safety Device
Manufactured for Amerinet Choice by
AMPHASTAR PHARMACEUTICALS, INC.
Rancho Cucamonga, CA 91730, U.S.A.
Made in U.S.A.
© 2012
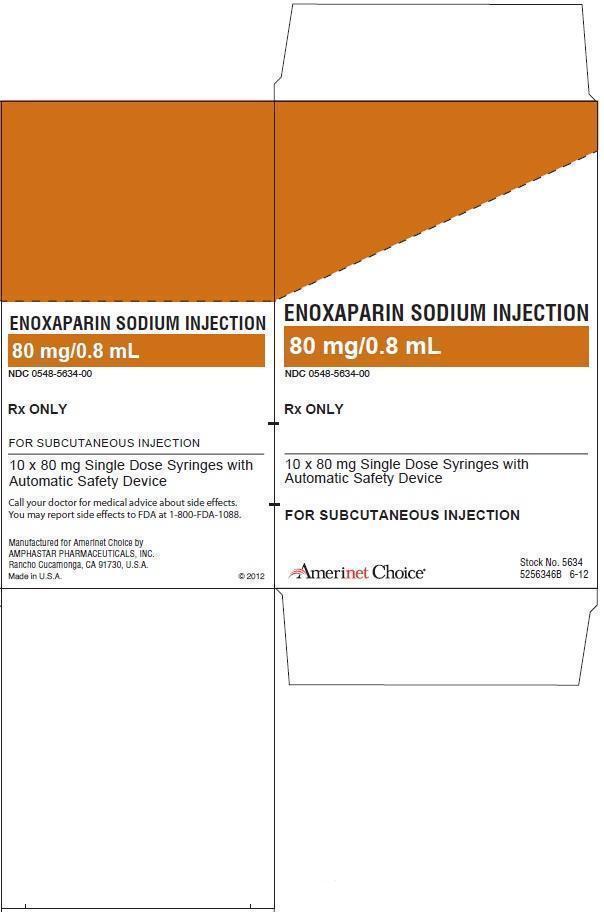

-
PRINCIPAL DISPLAY PANEL: 80 mg Syringe Peel Lid
Manufactured for Amerinet Choice by
AMPHASTAR PHARMACEUTICALS, INC.
Rancho Cucamonga, CA 91730, U.S.A.
Remove syringe by peeling lid completely off. Then gently lift straight up by grasping middle of syringe. Do not remove syringe by pulling on plunger. See www.amphastar.com
ENOXAPARIN SODIUM INJECTION
80 mg/0.8 mL
Each 0.8 mL contains 80 mg of enoxaparin sodium derived from porcine mucosa in Water for Injection. See insert for directions for use.
FOR SUBCUTANEOUS INJECTION
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [See USP Controlled Room Temperature].
WARNING: KEEP OUT OF REACH OF CHILDREN
Each 0.025 mL graduation equals 2.5 mg Enoxaparin Sodium Injection
RX ONLY
8856346C 11-12
Stock No. 5634
NDC: 0548-5634-00
1 Single Dose Prefilled Syringe with Automatic Safety Device
One 0.8 mL Syringe
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.

-
PRINCIPAL DISPLAY PANEL: 10 x 100 mg Syringe Carton
ENOXAPARIN SODIUM INJECTION
100 mg / mL
NDC: 0548-5635-00
1 mL Syringe
Rx ONLY
FOR SUBCUTANEOUS INJECTION
10 x 100 mg Single Dose Syringes with Automatic Safety Device
Manufactured for Amerinet Choice by
AMPHASTAR PHARMACEUTICALS, INC.
Rancho Cucamonga, CA 91730, U.S.A.
Made in U.S.A.
© 2012
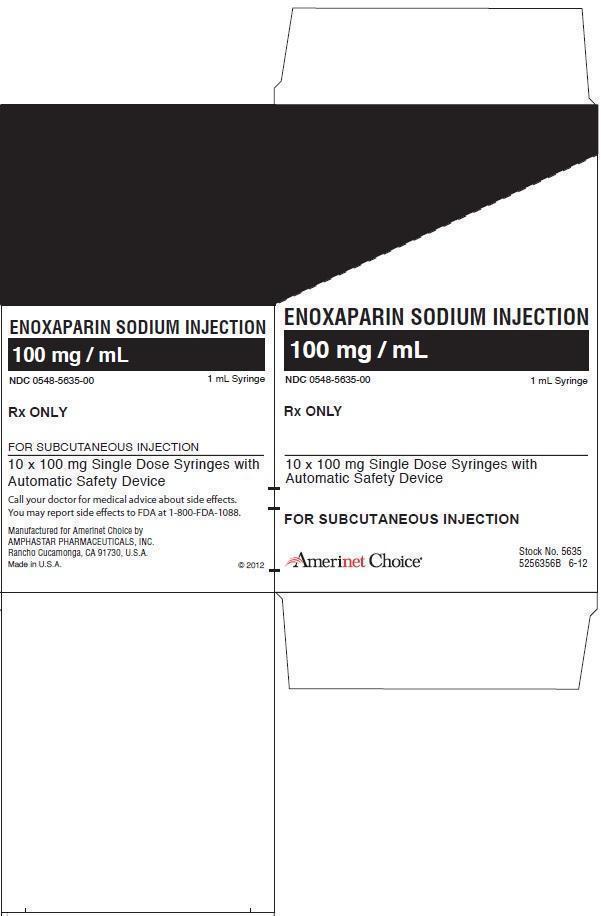

-
PRINCIPAL DISPLAY PANEL: 80 mg Syringe Peel Lid
Manufactured for Amerinet Choice by
AMPHASTAR PHARMACEUTICALS, INC.
Rancho Cucamonga, CA 91730, U.S.A.
Remove syringe by peeling lid completely off. Then gently lift straight up by grasping middle of syringe. Do not remove syringe by pulling on plunger. See www.amphastar.com
ENOXAPARIN SODIUM INJECTION
100 mg/mL
Each mL contains 100 mg of enoxaparin sodium derived from porcine mucosa in Water for Injection. See insert for directions for use.
FOR SUBCUTANEOUS INJECTION
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [See USP Controlled Room Temperature].
WARNING: KEEP OUT OF REACH OF CHILDREN
Each 0.025 mL graduation equals 2.5 mg Enoxaparin Sodium Injection
RX ONLY
8856356C 11-12
Stock No. 5635
NDC: 0548-5635-00
1 Single Dose Prefilled Syringe with Automatic Safety Device
One 0.6 mL Syringe
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.

-
PRINCIPAL DISPLAY PANEL: 10 x 120 mg Syringe Carton
ENOXAPARIN SODIUM INJECTION
120 mg/0.8 mL
NDC: 0548-5636-00
Rx ONLY
FOR SUBCUTANEOUS INJECTION
10 x 120 mg Single Dose Syringes with Automatic Safety Device
Manufactured for Amerinet Choice by
AMPHASTAR PHARMACEUTICALS, INC.
Rancho Cucamonga, CA 91730, U.S.A.
Made in U.S.A.
© 2012

-
PRINCIPAL DISPLAY PANEL: 120 mg Syringe Peel Lid
Manufactured for Amerinet Choice by
AMPHASTAR PHARMACEUTICALS, INC.
Rancho Cucamonga, CA 91730, U.S.A.
Remove syringe by peeling lid completely off. Then gently lift straight up by grasping middle of syringe. Do not remove syringe by pulling on plunger. See www.amphastar.com
ENOXAPARIN SODIUM INJECTION
120 mg/0.8 mL
Each 0.8 mL contains 120 mg of enoxaparin sodium derived from porcine mucosa in Water for Injection. See insert for directions for use.
FOR SUBCUTANEOUS INJECTION
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [See USP Controlled Room Temperature].
WARNING: KEEP OUT OF REACH OF CHILDREN
Each 0.02 mL graduation equals 3 mg Enoxaparin Sodium Injection
RX ONLY
8856366C 11-12
Stock No. 5636
NDC: 0548-5636-00
1 Single Dose Prefilled Syringe with Automatic Safety Device
One 0.8 mL Syringe
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.

-
PRINCIPAL DISPLAY PANEL: 10 x 150 mg Syringe Carton
ENOXAPARIN SODIUM INJECTION
150 mg / mL
NDC: 0548-5637-00
1 mL Syringe
Rx ONLY
FOR SUBCUTANEOUS INJECTION
10 x 150 mg Single Dose Syringes with Automatic Safety Device
Manufactured for Amerinet Choice by
AMPHASTAR PHARMACEUTICALS, INC.
Rancho Cucamonga, CA 91730, U.S.A.
Made in U.S.A.
© 2012

-
PRINCIPAL DISPLAY PANEL: 150 mg Syringe Peel Lid
Manufactured for Amerinet Choice by
AMPHASTAR PHARMACEUTICALS, INC.
Rancho Cucamonga, CA 91730, U.S.A.
Remove syringe by peeling lid completely off. Then gently lift straight up by grasping middle of syringe. Do not remove syringe by pulling on plunger. See www.amphastar.com
ENOXAPARIN SODIUM INJECTION
150 mg/mL
Each mL contains 150 mg of enoxaparin sodium derived from porcine mucosa in Water for Injection. See insert for directions for use.
FOR SUBCUTANEOUS INJECTION
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [See USP Controlled Room Temperature].
WARNING: KEEP OUT OF REACH OF CHILDREN
Each 0.02 mL graduation equals 3 mg Enoxaparin Sodium Injection
RX ONLY
8856376C 11-12
Stock No. 5637
NDC: 0548-5637-00
1 Single Dose Prefilled Syringe with Automatic Safety Device
One 1 mL Syringe
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
-
INGREDIENTS AND APPEARANCE
ENOXAPARIN SODIUM
enoxaparin sodium injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0548-5631 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Enoxaparin sodium (UNII: 8NZ41MIK1O) (enoxaparin - UNII:E47C0NF7LV) Enoxaparin sodium 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0548-5631-00 10 in 1 CARTON 09/19/2011 1 0.3 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076684 09/19/2011 ENOXAPARIN SODIUM
enoxaparin sodium injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0548-5632 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Enoxaparin sodium (UNII: 8NZ41MIK1O) (enoxaparin - UNII:E47C0NF7LV) Enoxaparin sodium 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0548-5632-00 10 in 1 CARTON 09/19/2011 1 0.4 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076684 09/19/2011 ENOXAPARIN SODIUM
enoxaparin sodium injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0548-5633 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Enoxaparin sodium (UNII: 8NZ41MIK1O) (enoxaparin - UNII:E47C0NF7LV) Enoxaparin sodium 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0548-5633-00 10 in 1 CARTON 09/19/2011 1 0.6 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076684 09/19/2011 ENOXAPARIN SODIUM
enoxaparin sodium injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0548-5634 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Enoxaparin sodium (UNII: 8NZ41MIK1O) (enoxaparin - UNII:E47C0NF7LV) Enoxaparin sodium 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0548-5634-00 10 in 1 CARTON 09/19/2011 1 0.8 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076684 09/19/2011 ENOXAPARIN SODIUM
enoxaparin sodium injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0548-5635 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Enoxaparin sodium (UNII: 8NZ41MIK1O) (enoxaparin - UNII:E47C0NF7LV) Enoxaparin sodium 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0548-5635-00 10 in 1 CARTON 09/19/2011 1 1 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076684 09/19/2011 ENOXAPARIN SODIUM
enoxaparin sodium injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0548-5636 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Enoxaparin sodium (UNII: 8NZ41MIK1O) (enoxaparin - UNII:E47C0NF7LV) Enoxaparin sodium 150 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0548-5636-00 10 in 1 CARTON 09/19/2011 1 0.8 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076684 09/19/2011 ENOXAPARIN SODIUM
enoxaparin sodium injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0548-5637 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Enoxaparin sodium (UNII: 8NZ41MIK1O) (enoxaparin - UNII:E47C0NF7LV) Enoxaparin sodium 150 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0548-5637-00 10 in 1 CARTON 09/19/2011 1 1 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076684 09/19/2011 Labeler - Amphastar Pharmaceuticals, Inc. (024736733) Establishment Name Address ID/FEI Business Operations Amphastar Pharmaceuticals, Inc. 024736733 analysis(0548-5631, 0548-5632, 0548-5634, 0548-5635, 0548-5636, 0548-5637) , manufacture(0548-5631, 0548-5632, 0548-5633, 0548-5634, 0548-5635, 0548-5636, 0548-5637) , label(0548-5631, 0548-5633, 0548-5633, 0548-5634, 0548-5635, 0548-5636, 0548-5637)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.