SPA DRY HEEL FOOT RELIEF- dimethicone cream
Spa Dry Heel Foot Relief by
Drug Labeling and Warnings
Spa Dry Heel Foot Relief by is a Otc medication manufactured, distributed, or labeled by Jafra Cosmetics International Inc, Distribuidora Comercial Jafra, S.A. de C.V.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
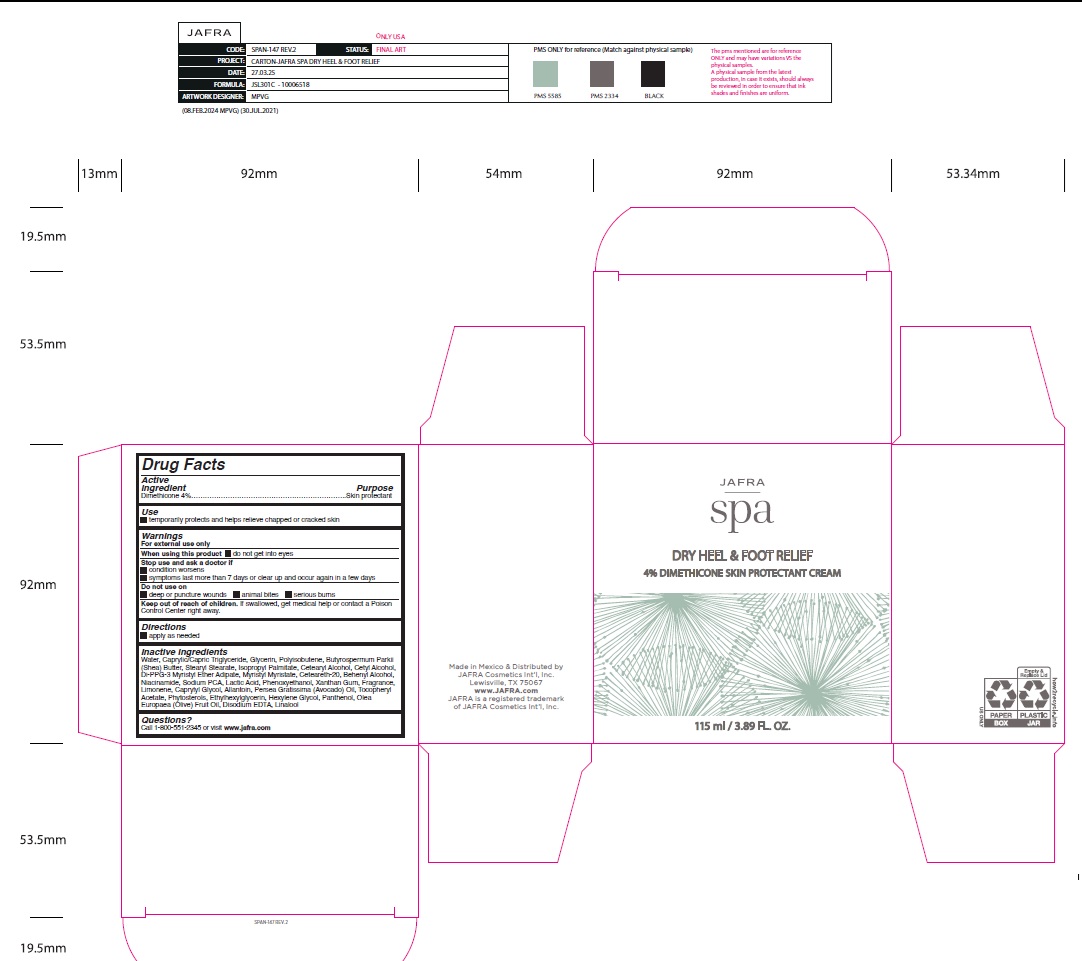
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
When using this product● do not get into eyes
Stop use and ask a doctor● condition worsens ● symptoms last more than 7 days or clear up and occur again in a few days
Do not use on● deep or puncture wounds ● animal bites ● serious burns
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away. - Directions
- KEEP OUT OF REACH OF CHILDREN
-
Inactive ingredients
Water, Caprylic/Capric Triglyceride, Glycerin, Polyisobutene, Butyrospermum Parkii (Shea) Butter, Stearyl Stearate, Isopropyl Palmitate, Cetearyl Alcohol, Cetyl Alcohol, Di-PPG-3 Myristyl Ether Adipate, Myristyl Myristate, Ceteareth-20, Behenyl Alcohol, Niacinamide, Sodium PCA, Lactic Acid, Phenoxyethanol , Xanthan Gum, Fragrance, Limonene, Caprylyl Glycol, Allantoin, Persea Gratissima (Avocado) Oil, Tocopheryl Acetate, Phytosterols, Ethylhexylglycerin, Hexylene Glycol, Panthenol, Olea Europaea (Olive) Fruit Oil, Disodium EDTA, Linalool
- SPL UNCLASSIFIED SECTION
- Product label
-
INGREDIENTS AND APPEARANCE
SPA DRY HEEL FOOT RELIEF
dimethicone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 68828-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 4 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) GLYCERIN (UNII: PDC6A3C0OX) POLYISOBUTYLENE (55000 MW) (UNII: TQ77WR8A02) SHEA BUTTER (UNII: K49155WL9Y) STEARYL STEARATE (UNII: 5WX2EGD0DK) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETYL ALCOHOL (UNII: 936JST6JCN) DI-PPG-3 MYRISTYL ETHER ADIPATE (UNII: T32481VTXW) MYRISTYL MYRISTATE (UNII: 4042ZC00DY) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) DOCOSANOL (UNII: 9G1OE216XY) NIACINAMIDE (UNII: 25X51I8RD4) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) OLIVE OIL (UNII: 6UYK2W1W1E) PHENOXYETHANOL (UNII: HIE492ZZ3T) XANTHAN GUM (UNII: TTV12P4NEE) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ALLANTOIN (UNII: 344S277G0Z) AVOCADO OIL (UNII: 6VNO72PFC1) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HEXYLENE GLYCOL (UNII: KEH0A3F75J) PANTHENOL (UNII: WV9CM0O67Z) EDETATE DISODIUM (UNII: 7FLD91C86K) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68828-002-01 1 in 1 CARTON 06/01/2022 1 115 mL in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 06/01/2022 Labeler - Distribuidora Comercial Jafra, S.A. de C.V. (951612777) Registrant - Jafra Cosmetics International Inc (031183599) Establishment Name Address ID/FEI Business Operations Distribuidora Comercial Jafra, S.A. de C.V. 951612777 manufacture(68828-002)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.