VOYDEYA- danicopan tablet, film coated VOYDEYA- danicopan kit
Voydeya by
Drug Labeling and Warnings
Voydeya by is a Prescription medication manufactured, distributed, or labeled by Alexion Pharmaceuticals Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use VOYDEYA safely and effectively. See full prescribing information for VOYDEYA.
VOYDEYA™ (danicopan) tablets, for oral use
Initial U.S. Approval: 2024WARNING: SERIOUS INFECTIONS CAUSED BY ENCAPSULATED BACTERIA
See full prescribing information for complete boxed warning.
VOYDEYA increases the risk of serious and life-threatening infections, caused by encapsulated bacteria, including Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae type B (5.1).
- Complete or update vaccination for encapsulated bacteria at least 2 weeks prior to the first dose of VOYDEYA, unless the risks of delaying VOYDEYA outweigh the risk of developing a serious infection. Comply with the most current Advisory Committee on Immunization Practices (ACIP) recommendations for vaccinations against encapsulated bacteria in patients receiving a complement inhibitor (5.1).
- Patients receiving VOYDEYA are at increased risk for invasive disease caused by encapsulated bacteria, even if they develop antibodies following vaccination. Monitor patients for early signs and symptoms of serious infections and evaluate immediately if infection is suspected (5.1).
VOYDEYA is available only through a restricted program called VOYDEYA REMS (5.2).
INDICATIONS AND USAGE
VOYDEYA is a complement factor D inhibitor indicated as add-on therapy to ravulizumab or eculizumab for the treatment of extravascular hemolysis (EVH) in adults with paroxysmal nocturnal hemoglobinuria (PNH) (1).
Limitations of Use
VOYDEYA has not been shown to be effective as monotherapy and should only be prescribed as an add-on to ravulizumab or eculizumab.
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Tablets: 50 mg and 100 mg (3)
CONTRAINDICATIONS
Initiation in patients with unresolved serious infection caused by encapsulated bacteria (4).
WARNINGS AND PRECAUTIONS
- Hepatic Enzyme Increases: Assess liver enzymes before treatment initiation and periodically during treatment. Consider treatment interruption or discontinuation if elevations are clinically significant or if the patient becomes symptomatic (5.3).
- Hyperlipidemia: Monitor serum lipids periodically during treatment and initiate cholesterol-lowering medication if indicated (5.4).
ADVERSE REACTIONS
Most frequent adverse reaction (incidence ≥10%) was headache (6).
To report SUSPECTED ADVERSE REACTIONS, contact Alexion Pharmaceuticals, Inc. at 1-844-259-6783 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- BCRP substrates: Monitor patients more frequently for adverse reactions and consider dose reduction of the BCRP substrate drug. For rosuvastatin, the dose should not exceed 10 mg once daily (7.1).
- P-gp substrates: Dose adjustment might be necessary for P-gp substrates where minimal concentration changes may lead to serious adverse reactions (7.2).
USE IN SPECIFIC POPULATIONS
Hepatic Impairment: Avoid use in patients with severe hepatic impairment (Child-Pugh C) (8.6).
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 3/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: SERIOUS INFECTIONS CAUSED BY ENCAPSULATED BACTERIA
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Vaccination and Prophylaxis for Encapsulated Bacterial Infections
2.2 Recommended Dosage
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Serious Infections Caused by Encapsulated Bacteria
5.2 VOYDEYA REMS
5.3 Hepatic Enzyme Increases
5.4 Monitoring of PNH Manifestations After VOYDEYA Discontinuation
5.5 Hyperlipidemia
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 BCRP Substrates
7.2 P-gp Substrates
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: SERIOUS INFECTIONS CAUSED BY ENCAPSULATED BACTERIA
VOYDEYA, a complement inhibitor, increases the risk of serious infections, especially those caused by encapsulated bacteria, such as Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae type B [see Warnings and Precautions (5.1)]. Life-threatening and fatal infections with encapsulated bacteria have occurred in patients treated with complement inhibitors. These infections may become rapidly life-threatening or fatal if not recognized and treated early.
- Complete or update vaccination for encapsulated bacteria specifically, Neisseria meningitidis and Streptococcus pneumoniae at least 2 weeks prior to the first dose of VOYDEYA, unless the risks of delaying therapy with VOYDEYA outweigh the risk of developing a serious infection. Comply with the most current Advisory Committee on Immunization Practices (ACIP) recommendations for vaccinations against encapsulated bacteria in patients receiving a complement inhibitor. See Warnings and Precautions (5.1) for additional guidance on the management of the risk of serious infections caused by encapsulated bacteria.
- Patients receiving VOYDEYA are at increased risk for invasive disease caused by encapsulated bacteria, even if they develop antibodies following vaccination. Monitor patients for early signs and symptoms of serious infections and evaluate immediately if infection is suspected.
Because of the risk of serious infections caused by encapsulated bacteria, VOYDEYA is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the VOYDEYA REMS [see Warnings and Precautions (5.2)].
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Vaccination and Prophylaxis for Encapsulated Bacterial Infections
Vaccinate patients against encapsulated bacteria, including Neisseria meningitidis (serogroups A, C, W, Y, and B) and Streptococcus pneumoniae according to current ACIP recommendations at least 2 weeks prior to initiation of VOYDEYA.
If urgent VOYDEYA therapy is indicated in a patient who is not up to date with vaccines for Neisseria meningitidis and Streptococcus pneumoniae according to ACIP recommendations, provide the patient with antibacterial drug prophylaxis and administer these vaccines as soon as possible [see Warnings and Precautions (5.1)].
Healthcare professionals who prescribe VOYDEYA must enroll in the VOYDEYA REMS [see Warnings and Precautions (5.2)].
2.2 Recommended Dosage
Starting Dose:
The recommended dosage of VOYDEYA is 150 mg three times a day administered orally.
VOYDEYA can be taken with or without food.
Dose Adjustment:
The dose can be increased to 200 mg three times a day if the patient's hemoglobin (Hgb) level has not increased by greater than 2 g/dL after 4 weeks of therapy, if the patient required a transfusion during the previous 4 weeks, or to achieve an appropriate Hgb response based on clinical judgement.
Missed Doses
A patient who misses a dose of VOYDEYA should take it as soon as they remember unless it is within 3 hours prior to their next dose, in which case the patient should skip the missed dose and take VOYDEYA at the next regularly scheduled time. Patients should not take two or more doses of VOYDEYA at the same time.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
VOYDEYA is contraindicated for initiation in patients with unresolved serious infection caused by encapsulated bacteria, including Neisseria meningitidis, Streptococcus pneumoniae, or Haemophilus influenzae type B [see Warnings and Precautions (5.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Serious Infections Caused by Encapsulated Bacteria
VOYDEYA, a complement inhibitor, increases a patient's susceptibility to serious, life-threatening, or fatal infections caused by encapsulated bacteria including Neisseria meningitidis (caused by any serogroup, including non-groupable strains), Streptococcus pneumoniae, and Haemophilus influenzae type B. Life-threatening and fatal infections with encapsulated bacteria have occurred in both vaccinated and unvaccinated patients treated with complement inhibitors. The initiation of VOYDEYA treatment is contraindicated in patients with unresolved serious infections caused by encapsulated bacteria.
Complete or update vaccination against encapsulated bacteria, specifically Neisseria meningitidis and Streptococcus pneumoniae at least 2 weeks prior to administration of the first dose of VOYDEYA, according to the current ACIP recommendations for patients receiving a complement inhibitor. Revaccinate patients in accordance with ACIP recommendations considering the duration of therapy with VOYDEYA. Note that ACIP recommends an administration schedule in patients receiving complement inhibitors that differs from the administration schedule in the vaccine prescribing information. If urgent VOYDEYA therapy is indicated in a patient who is not up to date with vaccines against encapsulated bacteria according to ACIP recommendations, provide the patient with antibacterial drug prophylaxis and administer these vaccines as soon as possible. Various durations and regimens of antibacterial drug prophylaxis have been considered, but the optimal durations and drug regimens for prophylaxis and their efficacy have not been studied in unvaccinated or vaccinated patients receiving complement inhibitors, including VOYDEYA. The benefits and risks of treatment with VOYDEYA, as well as the benefits and risks of antibacterial drug prophylaxis in unvaccinated or vaccinated patients, must be considered against the known risks for serious infections caused by encapsulated bacteria.
Vaccination does not eliminate the risk of serious encapsulated bacterial infections, despite development of antibodies following vaccination. Closely monitor patients for early signs and symptoms of serious infection and evaluate patients immediately if an infection is suspected. Inform patients of these signs and symptoms and instruct patients to seek immediate medical care if these signs and symptoms occur. Promptly treat known infections. Serious infection may become rapidly life-threatening or fatal if not recognized and treated early. Consider interruption of VOYDEYA in patients who are undergoing treatment for serious infections.
VOYDEYA is available only through a restricted program under a REMS [see Warnings and Precautions (5.2)].
5.2 VOYDEYA REMS
VOYDEYA is available only through a restricted program under a REMS called VOYDEYA REMS, because of the risk of serious infections caused by encapsulated bacteria [see Warnings and Precautions (5.1)].
Notable requirements of the VOYDEYA REMS include the following:
- Prescribers must enroll in the REMS.
- Prescribers must counsel patients about the risk of serious infections caused by encapsulated bacteria.
- Prescribers must provide patients with the REMS educational materials.
- Prescribers must assess patient vaccination status for vaccines against encapsulated bacteria and vaccinate if needed according to current ACIP recommendations two weeks prior to the first dose of VOYDEYA.
- Prescribers must provide a prescription for antibacterial drug prophylaxis if treatment must be started urgently, and the patient is not up to date with vaccines against encapsulated bacteria according to current ACIP recommendations at least two weeks prior to the first dose of VOYDEYA.
- Pharmacies that dispense VOYDEYA must be certified in the VOYDEYA REMS and must verify prescribers are certified.
- Patients must receive counseling from the prescriber about the need to receive vaccinations against encapsulated bacteria per ACIP recommendations, the need to take antibiotics as directed by the prescriber, and the early signs and symptoms of serious infections.
- Patients must be instructed to carry the Patient Safety Card with them at all times during treatment and for 1 week following the last dose of VOYDEYA.
Further information is available by telephone: 1-888-765-4747 or online at www.VoydeyaREMS.com.
5.3 Hepatic Enzyme Increases
Hepatic enzyme elevations have been observed in patients treated with VOYDEYA [see Adverse Reactions (6.1)]. Fourteen percent of patients receiving VOYDEYA in Study ALXN2040-PNH-301 had elevations in serum alanine aminotransferase (ALT). ALT elevations > 3 × the upper limit of normal (ULN) and ≤ 5 × ULN occurred in 9% of VOYDEYA-treated patients, and ALT elevations > 5 × ULN and ≤ 10 × ULN occurred in 5% of VOYDEYA-treated patients.
Assess liver enzyme test results prior to the initiation of VOYDEYA and periodically during treatment. Consider treatment interruption or discontinuation if elevations are clinically significant or if the patient becomes symptomatic. VOYDEYA has not been studied in patients with severe hepatic impairment [see Use in Specific Populations (8.7)].
5.4 Monitoring of PNH Manifestations After VOYDEYA Discontinuation
After discontinuing treatment with VOYDEYA, closely monitor patients for at least 2 weeks after the last dose for signs and symptoms of hemolysis. If discontinuation of VOYDEYA is necessary, continue background treatment with ravulizumab or eculizumab or consider alternative therapy if necessary. The signs and symptoms of hemolysis may include a sudden decrease in hemoglobin or fatigue.
If hemolysis occurs after discontinuation of VOYDEYA, consider restarting treatment with VOYDEYA if appropriate.
5.5 Hyperlipidemia
VOYDEYA increases total cholesterol and LDL-cholesterol.
Of the 50 VOYDEYA-treated patients who had a normal total cholesterol level at baseline in Study ALXN2040-PNH-301, 30% developed Grade 1 hypercholesterolemia. Of the 6 VOYDEYA treated patients who had Grade 1 hypercholesterolemia at baseline in Study ALXN2040-PNH-301, 1 patient experienced increased total cholesterol that worsened to Grade 2. Of the 54 VOYDEYA-treated patients who had LDL-cholesterol ≤130 mg/dL at baseline in Study ALXN2040-PNH-301, 13% developed LDL-cholesterol >130-160 mg/dL and 9% developed LDL-cholesterol >160-190 mg/dL.
Some patients required cholesterol-lowering medications.
Monitor serum lipid parameters periodically during treatment with VOYDEYA and initiate cholesterol lowering medication, if indicated.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed in greater detail in other sections of the labeling:
- Serious Infections Caused by Encapsulated Bacteria [see Warnings and Precautions (5.1)]
- Hepatic Enzyme Increases [see Warnings and Precautions (5.3)]
- Hyperlipidemia [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of VOYDEYA was evaluated in 86 adults with PNH in Study ALXN2040-PNH-301 [see Clinical Studies (14)]. Study ALXN2040-PNH-301 enrolled adults with PNH with clinically significant EVH who had been treated with a stable dose of ravulizumab or eculizumab for at least the previous 6 months. Patients were randomly assigned 2:1 to receive double-blind VOYDEYA 150 mg (n=57) or placebo (n=29) orally three times a day in addition to ravulizumab or eculizumab for 12 weeks. Patients received either US-approved or non-US-approved ravulizumab or eculizumab in the trial. Among patients who were randomized to receive VOYDEYA, 84% were exposed for at least 12 weeks.
Serious adverse reactions were reported in 5% of patients who received VOYDEYA and included pancreatitis, cholecystitis, and blood bilirubin increased. No specific serious adverse reaction was reported in more than 1 patient treated with VOYDEYA.
Permanent discontinuation of VOYDEYA due to an adverse reaction occurred in 5% of patients and included 1 patient with blood bilirubin increase and pancreatitis, 1 patient with hepatic enzyme increased, and 1 patient with ALT increased and aspartate aminotransferase increased. Dosage reduction due to an adverse reaction occurred in 1 patient and the adverse reaction was COVID-19.
Among the 57 patients treated with VOYDEYA in Study ALXN2040-PNH-301, the most common adverse reaction (≥10%) was headache.
Table 1 describes adverse reactions reported in ≥5% of patients treated with VOYDEYA and greater than placebo in the randomized, controlled period of Study ALXN2040-PNH-301.
Table 1 Adverse Reactions Reported in ≥5% of VOYDEYA-Treated Patients with PNH and Greater than Placebo Adverse Reactions* VOYDEYA
(with ravulizumab or eculizumab)
N = 57Placebo
(ravulizumab or eculizumab only)
N = 29n (%) n (%) - * Common Toxicity Criteria Adverse Events (CTCAE)
- † Represents a composite of multiple, related adverse reactions
Headache 6 (11) 3 (10) Vomiting† 4 (7) 0 (0) Pyrexia† 4 (7) 0 (0) Alanine aminotransferase increased 3 (5) 1 (3) Hypertension 3 (5) 1 (3) Pain in extremity 3 (5) 0 (0) Clinically relevant adverse reactions in <5% of patients include increased serum triglycerides.
-
7 DRUG INTERACTIONS
7.1 BCRP Substrates
Danicopan is a Breast Cancer Resistance Protein (BCRP) inhibitor. Concomitant use of VOYDEYA with a BCRP substrate increases the plasma concentrations of the BCRP substrate [see Clinical Pharmacology (12.3)], which may increase the risk for adverse reactions associated with the BCRP substrate. If used together, monitor patients more frequently for adverse reactions associated with the BCRP substrate, and consider dose reduction of the BCRP substrate according to its prescribing information.
Rosuvastatin
Danicopan significantly increased rosuvastatin exposure. The dose of rosuvastatin should not exceed 10 mg once daily when concomitantly used with VOYDEYA [see Clinical Pharmacology (12.3)].
7.2 P-gp Substrates
Danicopan is an inhibitor of P-glycoprotein (P-gp). Concomitant administration of VOYDEYA with a P-gp substrate may increase the plasma concentration of the P-gp substrate. Dose adjustment might be necessary for P-gp substrates where minimal concentration changes may lead to serious adverse reactions [see Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on VOYDEYA use in pregnant individuals to evaluate for a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. There are risks to the mother and fetus associated with untreated PNH in pregnancy (see Clinical Considerations). The use of VOYDEYA in pregnant women or women planning to become pregnant may be considered following an assessment of the risks and benefits.
In animal reproduction studies, oral administration of danicopan to pregnant New Zealand White (NZW) rabbits and Wistar Hans (WH) rats during organogenesis at exposures 18 or 25-times, respectively, above the human exposure at the maximum recommended human dose (MRHD) of 200 mg three times a day (based on AUC) resulted in no adverse developmental effects (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Fetal/Neonatal Risk
PNH in pregnancy is associated with adverse maternal outcomes, including worsening cytopenias, thrombotic events, infections, bleeding, miscarriages, and increased maternal mortality, and adverse fetal outcomes, including fetal death and premature delivery.
Data
Animal Data
There were no effects on early embryonic development and fetal development in NZW rabbits (where danicopan is pharmacodynamically active) up to a mean maternal systemic exposure 18-times the exposure at the MRHD (based on AUC) or during post-natal development up to a mean maternal systemic exposure 9-times the exposure at the MRHD (based on AUC). In WH rats (where danicopan lacks pharmacodynamic activity), there were no effects on embryo-fetal development up to a mean maternal exposure 25-times the exposure at the MRHD (based on AUC).
8.2 Lactation
Risk Summary
There are no data on the presence of danicopan in human milk, the effects on the breastfed child, or the effect on milk production. Danicopan is present in animal milk. When a drug is present in animal milk, it is likely that the drug will be present in human milk.
Because of the potential for serious adverse reactions in the breastfed child, including serious infections with encapsulated bacteria and liver enzyme increases, advise patients not to breastfeed during treatment with VOYDEYA, and for 3-days after the last dose.
Data
Animal Data
Danicopan was excreted into the milk of lactating rabbits following oral administration from lactation day 4 to lactation day 10, with mean milk concentrations at approximately 2 hours following dose administration 5- and 3.5-times higher than the mean maternal plasma concentrations at 50 and 250 mg/kg/day, respectively. Mean milk concentrations in dams were 19- and 43-times higher than the systemic exposure at the MRHD (based on rabbit concentration at 2 hours vs. human Cmax).
8.4 Pediatric Use
Safety and effectiveness of VOYDEYA for the treatment of PNH in pediatric patients have not been established.
8.5 Geriatric Use
There were 22 patients 65 years of age and older in the clinical studies for PNH [see Clinical Studies (14)]. Of the total number of VOYDEYA-treated patients in these studies, 16 (28.1%) were 65 years of age and older, and 7 (12.3%) were 75 years of age and older. Clinical studies of VOYDEYA did not include sufficient numbers of subjects aged 65 years and over to determine whether they respond differently from younger subjects.
8.6 Hepatic Impairment
No dose adjustment is required in patients with mild to moderate hepatic impairment (Child-Pugh Class A and B). Studies have not been conducted in patients with severe hepatic impairment, therefore, avoid use of VOYDEYA in this patient population [see Warnings and Precautions (5.3)].
-
10 OVERDOSAGE
Serum ALT elevations occurred after treatment cessation without a taper in 2 healthy subjects who received danicopan 500 mg and 800 mg twice a day. These abnormal ALT findings were transient, with no evidence of hepatic function abnormality and resolved spontaneously. In case of overdose, elevations in liver enzymes may occur. General supportive measures are recommended. It is not known if VOYDEYA can be removed by dialysis.
-
11 DESCRIPTION
Danicopan is a small molecule complement Factor D inhibitor. Its chemical name is (2S,4R)-1-{[3-acetyl-5-(2-methylpyrimidin-5-yl)-1H-indazol-1-yl] acetyl}-N-(6-bromopyridin-2-yl)-4-fluoropyrrolidine-2-carboxamide. Its molecular formula is C26H23BrFN7O3 and its molecular weight is 580.4. Danicopan has the following structural formula:
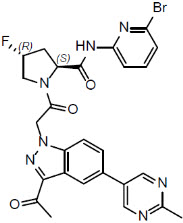
Danicopan is a white/off-white to pale yellow powder. In aqueous solutions, danicopan is considered slightly soluble at pH 1.2 and insoluble from pH 4 to pH 7.
Danicopan tablets are available as white to off-white, round, film-coated, immediate release tablets in strengths of 50 mg and 100 mg, intended for oral administration. Each tablet contains the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, hypromellose acetate succinate, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and sodium lauryl sulfate. The tablet coating components are polyethylene glycol, polyvinyl alcohol, talc, and titanium dioxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Danicopan binds reversibly to complement Factor D and selectively inhibits the alternative complement pathway. Danicopan prevents the cleavage of complement Factor B into the Ba and Bb fragments which are required for the formation of the alternative pathway (AP) complement component C3 convertase (C3bBb), the generation of downstream effectors including C3 fragment opsonization, and the amplification of the terminal pathway.
In PNH, intravascular hemolysis (IVH) is mediated by the terminal membrane attack complex (MAC), while extravascular hemolysis (EVH) is facilitated by C3 fragment opsonization. Danicopan acts proximally in the alternative pathway of the complement cascade to control preferentially C3 fragment-mediated EVH, while co-administered ravulizumab or eculizumab is anticipated to maintain control over MAC-mediated IVH.
12.2 Pharmacodynamics
Danicopan inhibits the AP of the complement system, as demonstrated by the decrease in ex vivo serum AP activity and in vivo plasma Bb concentration. Danicopan also reduces complement C3 fragment deposition on circulating red blood cells (RBCs) in PNH patients.
In patients with PNH undergoing treatment with ravulizumab or eculizumab, co-administration of VOYDEYA from 150 mg three times a day to 200 mg three times a day inhibited AP activity by >90%. Additionally, plasma Bb levels decreased by about 50% and the fraction of circulating PNH RBCs with measured C3 fragment deposition decreased by over 50%.
12.3 Pharmacokinetics
At the recommended dosages of 150 or 200 mg three times a day, the median systemic exposure of danicopan at steady state has a maximum plasma concentration (Cmax,ss) of 535 or 665 ng/mL, respectively, and has an area under the plasma drug concentration time curve (AUC24,ss) of 8180 or 10200 ng × h/mL, respectively.
Danicopan exposures at steady state generally increase in a dose-proportional manner from 150 mg three times a day to 200 mg three times a day. Danicopan systemic exposure reaches steady state in approximately 2 days. An approximately 2-fold accumulation of danicopan exposure is expected at steady state following thrice daily dosing compared to a single dose.
Absorption
The median time to maximum drug concentration (Tmax) is 3.7 hours following oral administration of 150 mg danicopan in patients with PNH.
Effect of Food
When the danicopan tablet was administered with a high-fat meal, danicopan AUC and Cmax were approximately 25%, and 93% higher, respectively, compared to the fasted state. Median time to maximum drug concentration (Tmax) was comparable when danicopan was administered in the fed or fasted state at approximately 3.0 and 2.5 hours, respectively.
Distribution
Plasma protein binding of danicopan is 91.5% to 94.3%. Danicopan is mainly distributed in plasma with a whole blood to plasma distribution ratio of 0.545. The apparent volume of distribution for a 75 kg person was 395 L.
Elimination
The mean half-life (t½) is 7.9 hours. The mean apparent clearance of danicopan is 63 L/h.
Specific Populations
No clinically significant differences in the pharmacokinetics of danicopan were observed based on sex, age (16.9 to 82 years), or race (Caucasians and Asians) based on population pharmacokinetic (PK) assessment.
Renal Impairment
Following oral administration of danicopan 200 mg in subjects with severe renal impairment (eGFR < 30 mL/min/1.73 m2), the extent of danicopan exposure (AUC0-inf) increased by 52% as compared to subjects with normal renal function. There was no clinically meaningful change in Cmax and Tmax.
Drug Interaction Studies
Clinical Studies
Effect of Danicopan on the Pharmacokinetics of Other Drugs:
Dedicated clinical drug interaction studies showed no clinically significant drug interactions with danicopan as an inhibitor or inducer of CYP2B6 (bupropion), CYP2C9 (warfarin), CYP2C19 (omeprazole), CYP3A4 (midazolam), and UGT1A1 and UGT2B7 (mycophenolic acid).
BCRP Substrates
Co-administration of a single oral dose of rosuvastatin 20 mg with danicopan dosed to steady state (200 mg three times a day for 4 days) resulted in increased rosuvastatin Cmax and AUC0-inf by 3.3-fold and 2.2-fold, respectively.
P-gp Substrates
Co-administration of a single oral dose of fexofenadine 180 mg with danicopan dosed to steady state (150 mg three times a day for 4 days) resulted in increased fexofenadine Cmax and AUC0-inf by 1.4-fold and 1.6-fold, respectively.
Co-administration of a single oral dose of tacrolimus 2 mg with danicopan dosed to steady state (200 mg three times a day for 5 days) resulted in increased tacrolimus Cmax and AUC0-inf by 1.1-fold and 1.5-fold, respectively.
In Vitro Studies
Non-CYP based metabolism is the predominant clearance pathway for danicopan. The minimal contribution of CYP metabolism in human hepatocytes is suggestive of a very low likelihood of danicopan as a victim of CYP-based drug-drug interactions.
Danicopan is a substrate of P-gp, but not a substrate of BCRP, Organic Anion Transporting Polypeptide 1B1 (OATP1B1), or OATP1B3. Danicopan is not an inducer of CYP1A2, CYP2B6 or CYP2C9.
Danicopan is an inhibitor of BCRP and P-gp, but not an inhibitor of transporters OATP1B1, OATP1B3, Organic Anion Transporter (OAT)1, OAT3, Organic Cation Transporter 2 (OCT2), or Multidrug And Toxin Extrusion 1 and 2K (MATE1 and MATE2-K).
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Danicopan was not carcinogenic in the 6-month carcinogenicity study in the TgRasH2 mouse model. Danicopan was not carcinogenic in the 2-year rat carcinogenicity study at exposures 15- to 23-times the exposure at the MRHD (based on AUC).
13.2 Animal Toxicology and/or Pharmacology
Ocular phototoxicity was observed in pigmented rats at systemic exposures 15-times and 28-times the exposure at the MRHD (based on AUC and Cmax, respectively). As danicopan is expected to accumulate in the eye, a risk of developing ocular phototoxicity cannot be excluded in patients on long-term danicopan therapy who are exposed to unprotected ultraviolet radiation for extended periods of time. The clinical significance of these findings is unknown.
-
14 CLINICAL STUDIES
Paroxysmal Nocturnal Hemoglobinuria (PNH)
The efficacy of VOYDEYA in adults with PNH and clinically significant EVH was assessed in a multiple-region, randomized, double-blind, placebo-controlled study (ALXN2040-PNH-301; NCT04469465). Clinically significant EVH was defined by anemia (hemoglobin [Hgb] ≤ 9.5 g/dL) with absolute reticulocyte count ≥ 120 × 109/L with or without transfusion support. The study enrolled patients with PNH who had been treated with a stable dose of ravulizumab or eculizumab for at least the previous 6 months.
VOYDEYA was administered orally at 150 mg three times a day, escalated to 200 mg three times a day depending on the clinical response.
Patients were vaccinated against meningococcal infection prior to or at the time of initiating treatment with VOYDEYA if vaccination status within 3 years could not be verified.
Patients were randomized to VOYDEYA or placebo in a 2:1 ratio for 12 weeks in addition to background ravulizumab or eculizumab treatment. After Week 12, all patients received VOYDEYA in combination with their background ravulizumab or eculizumab treatment up to Week 24. After Week 24, patients could enter a long-term extension period and continue to receive VOYDEYA with background ravulizumab or eculizumab.
Efficacy was based on the change in Hgb level from Baseline to Week 12. Other efficacy measures included the proportion of patients with Hgb increase of ≥ 2 g/dL at Week 12 in the absence of transfusions, the proportion of patients with transfusion avoidance through Week 12, the change from Baseline in Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue scores at Week 12, and change from Baseline in absolute reticulocyte count at Week 12. Transfusion avoidance was considered as achieved only by the patients who did not receive a transfusion and did not meet the protocol specified guidelines for transfusion from Baseline through Week 12.
A pre-specified interim analysis was performed when 63 participants reached the end (either completed or discontinued) of Week 12.
Baseline demographic and disease history characteristics were generally balanced between treatment groups.
Table 2 presents the baseline characteristics of the patients with PNH enrolled in the study.
Table 2 Baseline Characteristics in PNH Study (ALXN2040-PNH-301) Parameter Statistics VOYDEYA
(Add-on to ravulizumab or eculizumab)
N = 42Placebo
(Add-on to ravulizumab or eculizumab)
N = 21Abbreviations: FACIT = Functional Assessment of Chronic Illness Therapy; LDH = lactate dehydrogenase; N = number of patients; pRBC = packed red blood cell; SD = standard deviation - * Fatigue-related symptoms and impacts were assessed using a patient reported outcome instrument, FACIT-Fatigue (score range from 0 to 52 with higher scores indicating less fatigue).
Age (years) Mean (SD) 55 (16) 53 (14) Median 58 53 Min, max 25, 80 29, 75 Sex n (%) Male 19 (45.2) 7 (33.3) Female 23 (54.8) 14 (66.7) Race n (%) American Indian or Alaska Native 1 (2.4) 0 Asian 18 (42.9) 7 (33.3) Black or African American 1 (2.4) 0 White 19 (45.2) 9 (42.9) Other 1 (2.4) 0 Unknown 0 1 (4.8) Not Reported 2 (4.8) 4 (19.0) Ethnicity n (%) Hispanic or Latino 4 (9.5) 0 Not Hispanic or Latino 34 (81.0) 17 (81.0) Not reported 4 (9.5) 4 (19.0) Hemoglobin level (g/dL) Mean (SD) 7.7 (0.9) 7.7 (1.0) Reticulocyte count (109/L) N 42 20 Mean (SD) 236 (91) 241 (120) Number of patients with pRBC/whole blood transfusions within 24 weeks prior to first dose n (%) 38 (90) 17 (81) pRBC/whole blood Transfusions within 24 weeks prior to first dose Mean (SD) 2.5 (2.2) 2.6 (2.1) LDH (U/L) N 42 20 Mean (SD) 299 (106) 278 (68) FACIT-Fatigue score* Mean (SD) 33 (11) 34 (11) Background treatment with: n (%) Ravulizumab 27 (64.3) 10 (47.6) Eculizumab 15 (35.7) 11 (52.4) Efficacy was established based on demonstration of superiority of VOYDEYA in combination with ravulizumab or eculizumab compared to placebo in combination with ravulizumab or eculizumab in all efficacy measures with statistically significant results (Table 3).
Table 3 Efficacy Results for Patients with PNH (Study ALXN2040-PNH-301) VOYDEYA (Add-on to ravulizumab or eculizumab)
(N = 42)Placebo (Add-on to ravulizumab or eculizumab)
(N = 21)Abbreviations: CI = confidence interval; FACIT = Functional Assessment of Chronic Illness Therapy - * The model included the fixed, categorical effects of treatment group, study visit, and study visit-by-treatment group interaction, as well as the fixed, continuous covariate of baseline value and the randomization stratification factor of transfusion history. An unstructured covariance matrix was used to model the within-patient errors.
- † Statistical significance of the treatment group difference was evaluated via a re-randomization test method. The p-value for the re-randomization test was calculated as the number of re-randomized treatment group differences that were more extreme than the treatment group difference calculated under the actual randomization divided by the total number of simulated re-randomizations.
- ‡ The proportions were compared between treatment groups using the Cochrane-Mantel-Haenszel (CMH) test stratified by randomization stratification factors of transfusion history and screening Hgb level. The p-value was from the stratified CMH test.
- § The model included the fixed, categorical effects of treatment group, study visit, and study visit-by-treatment group interaction, as well as the fixed, continuous covariate of baseline value and the randomization stratification factors of transfusion history and screening Hgb level. An unstructured covariance matrix was used to model the within-patient errors. Fatigue-related symptoms and impacts were assessed using a patient reported outcome instrument, FACIT-Fatigue (score range from 0 to 52 with higher scores indicating less fatigue).
- ¶ The p-value was for the difference of LS mean from the mixed models for repeated measures (MMRM).
Change in Hemoglobin Level* Mean change from Baseline to Week 12 (g/dL) 2.9 0.5 Treatment difference 2.4 (95% CI: 1.7, 3.2) P-value 0.0007† Proportion of Patients with Hemoglobin Increase of ≥ 2 g/dL in the Absence of Transfusion ‡ At Week 12 (%) 59.5 0 Treatment difference 46.9 (95% CI: 29.2, 64.7) P-value < 0.0001 Proportion of Patients with Transfusion Avoidance‡ Through 12-Week Treatment Period (%) 83.3 38.1 Treatment difference 41.7 (95% CI: 22.7, 60.8) P-value 0.0004 Change in FACIT-Fatigue Score § Mean change from Baseline to Week 12 8.0 1.9 Treatment difference 6.1 (95% CI: 2.3, 9.9) P-value 0.002 ¶ Change in Absolute Reticulocyte Count § Mean change from Baseline to Week 12 (109/L) -84 4 Treatment difference -87 (95% CI: -118, -57) P-value < 0.0001 ¶ -
16 HOW SUPPLIED/STORAGE AND HANDLING
VOYDEYA (danicopan) tablets are available in the doses and packages listed in Table 4.
Table 4 VOYDEYA Tablet Presentations Dose Tablet Strength Film-Coated Tablet Markings Tablet Color/Shape Pack Size NDC Code Each carton contains two high density polyethylene bottles with desiccant and child resistant seal, with 180 tablets per carton: 150 mg 50 mg Debossed on one side with "DCN 50" White to off-white, round film-coated tablets One bottle with 90 × 50 mg per tablet
(25682-040-90)
One bottle with 90 × 100 mg per tablet
(25682-043-90)25682-046-92 100 mg Debossed on one side with "DCN 100" 200 mg 100 mg Debossed on one side with "DCN 100" White to off-white, round film-coated tablets Two bottles with 90 tablets per bottle:
90 × 100 mg per tablet
(25682-043-90)25682-043-92 Each carton contains four 7-day blister cards with 168 tablets per carton: 150 mg 50 mg Debossed on one side with "DCN 50" White to off-white, round film-coated tablets Four blister cards with 42 tablets per card:
21 × 50 mg per tablet and 21 × 100 mg per tablet
(25682-049-42)25682-049-04 100 mg Debossed on one side with "DCN 100" 200 mg 100 mg Debossed on one side with "DCN 100" White to off-white, round film-coated tablets Four blister cards with 42 tablets per card:
42 × 100 mg per tablet
(25682-043-42)25682-043-04 -
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Serious Infections Caused by Encapsulated Bacteria
Advise patients of the risk of serious infection. Inform patients of the need to complete or update their vaccinations against encapsulated bacteria at least 2 weeks prior to receiving the first dose of VOYDEYA or receive antibacterial drug prophylaxis if VOYDEYA treatment must be initiated immediately and they have not previously been vaccinated. Inform patients of the requirement to be revaccinated according to ACIP recommendations for encapsulated bacteria while on VOYDEYA therapy [see Warnings and Precautions (5.1)].
Inform patients that vaccination may not prevent serious infection and to seek immediate medical attention if the following signs or symptoms occur [see Warnings and Precautions (5.1)]:
- fever with or without chills
- fever and a rash
- fever with chest pain and cough
- fever with breathlessness/fast breathing
- fever with high heart rate
- headache with nausea or vomiting
- headache and a fever
- headache with a stiff neck or stiff back
- confusion
- body aches with flu-like symptoms
- clammy skin
- eyes sensitive to light
Inform patients that they will be given a Patient Safety Card for VOYDEYA that they should carry with them at all times during and for 1 week following treatment with VOYDEYA. This card describes symptoms which, if experienced, should prompt the patient to seek immediate medical evaluation.
VOYDEYA REMS
VOYDEYA is available only through a restricted program called VOYDEYA REMS [see Warnings and Precautions (5.2)].
Inform the patient of the following notable requirements:
- Patients must receive counseling about the risk of serious infections caused by encapsulated bacteria.
- Patients must receive written educational materials about this risk.
- Patients must be instructed to carry the Patient Safety Card with them at all times during treatment and for 1 week following the last dose of VOYDEYA.
- Patients must be instructed to complete or update vaccines against encapsulated bacteria per ACIP recommendations as directed by the prescriber prior to treatment with VOYDEYA.
- Patients must receive antibiotics as directed by the prescriber if they are not up to date on vaccinations against encapsulated bacteria and have to start VOYDEYA right away.
Importance of Adherence to Dosing Schedule
Inform patients with PNH of the importance of taking VOYDEYA as prescribed to minimize the risk of hemolysis.
Discontinuation
Inform patients with PNH that they may develop serious hemolysis due to PNH if VOYDEYA is discontinued and that they should be monitored by their healthcare providers for at least 2 weeks following discontinuation of VOYDEYA.
Inform patients who discontinue VOYDEYA to keep the Patient Safety Card with them for 1 week after the last dose of VOYDEYA. The increased risk of serious infection may continue for a few days after the last dose of VOYDEYA.
Hepatic Enzyme Elevations
Inform patients that elevation in liver enzymes have occurred in patients treated with VOYDEYA, and liver tests will be obtained before and during VOYDEYA treatment [see Warnings and Precautions (5.3)].
Hyperlipidemia
Inform patients that VOYDEYA may increase their cholesterol and that monitoring of these parameters will be needed periodically during treatment [see Warnings and Precautions (5.4)].
-
SPL UNCLASSIFIED SECTION
Manufactured for:
Alexion Pharmaceuticals, Inc.
121 Seaport Boulevard
Boston, MA 02210 USAThis product, or its use, may be covered by one or more US patents, including US Patent No. 9,796,741 B2 in addition to others including patents pending.
VOYDEYA is a trademark of Alexion Pharmaceuticals, Inc.
© 2024 Alexion Pharmaceuticals, Inc.
-
MEDICATION GUIDE
MEDICATION GUIDE
VOYDEYA™(voi-day-uh)
(danicopan)
tablets, for oral useThis Medication Guide has been approved by the U.S. Food and Drug Administration. Issued: 03/2024 What is the most important information I should know about VOYDEYA? VOYDEYA is a medicine that affects your immune system. VOYDEYA may lower the ability of your immune system to fight infections. -
VOYDEYA increases your chance of getting serious infections caused by encapsulated bacteria, including Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae type B. These serious infections may quickly become life-threatening and cause death if not recognized and treated early.
- You must complete or be up to date with the vaccines against Neisseria meningitidis and Streptococcus pneumoniae at least 2 weeks before your first dose of VOYDEYA.
- If you have not completed your vaccinations and VOYDEYA must be started right away, you should receive the required vaccinations as soon as possible.
- If you have not been vaccinated at least 2 weeks before your first VOYDEYA dose, and treatment with VOYDEYA must be started right away, you should also receive antibiotics to take for as long as your healthcare provider tells you.
- If you have been vaccinated against these bacteria in the past, you might need additional vaccinations before starting VOYDEYA. Your healthcare provider will decide if you need additional vaccinations.
- Vaccines do not prevent all infections caused by encapsulated bacteria. Call your healthcare provider or get emergency medical care right away if you have any of these signs and symptoms of a serious infection:
- fever with or without chills
- fever and a rash
- fever with chest pain and cough
- fever with breathlessness/fast breathing
- fever with high heart rate
- headache with nausea or vomiting
- headache and a fever
- headache with a stiff neck or stiff back
- confusion
- body aches with flu-like symptoms
- clammy skin
- eyes sensitive to light
Your healthcare provider will give you a Patient Safety Card about the risk of serious infections. Carry it with you at all times during treatment and for 1 week after your last VOYDEYA dose. Your risk of serious infections may continue for a few days after your last dose of VOYDEYA. If you get any of the symptoms listed on this card you should get medical help right away. It is important to show this card to any healthcare provider who treats you. This will help them diagnose and treat you quickly. -
VOYDEYA is only available through a program called the VOYDEYA REMS. Before you can take VOYDEYA, your healthcare provider must:
- enroll in the VOYDEYA REMS.
- counsel you about the risk of serious infections caused by certain bacteria.
- give you information about the symptoms of serious infections.
- make sure that you are vaccinated against serious infections caused by encapsulated bacteria and that you receive antibiotics if you need to start VOYDEYA right away and you are not up to date on your vaccinations.
- give you a Patient Safety Card about your risk of serious infections, as discussed above.
For more information about side effects, see "What are the possible side effects of VOYDEYA?" What is VOYDEYA? VOYDEYA is a prescription medicine used along with ravulizumab or eculizumab to treat breakdown of red blood cells that takes place outside of blood vessels (extravascular hemolysis), in adults with paroxysmal nocturnal hemoglobinuria (PNH).
It is not known if VOYDEYA is safe and effective in children.Who should not take VOYDEYA? Do not take VOYDEYA if you have a serious infection caused by encapsulated bacteria, including Neisseria meningitidis, Streptococcus pneumoniae, or Haemophilus influenzae type B when you are starting VOYDEYA treatment. Before taking VOYDEYA, tell your healthcare provider about all of your medical conditions, including if you: - have an infection or fever
- have liver problems
- are pregnant or plan to become pregnant. It is not known if VOYDEYA will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if VOYDEYA passes into your breast milk. Do not breastfeed during treatment with VOYDEYA and for 3 days after the last dose.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. VOYDEYA may affect the way other medicines work. Know the medicines you take and the vaccines you receive. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. How should I take VOYDEYA? - Take VOYDEYA exactly as your healthcare provider tells you to take it.
- Depending on the dose prescribed, the number of tablets is as follows:
- 150 mg dose: take 1 tablet of 100 mg and 1 tablet of 50 mg together 3 times a day.
- 200 mg dose: take 2 tablets of 100 mg 3 times a day.
- Do not change the dose or stop taking VOYDEYA unless your healthcare provider tells you.
- Take VOYDEYA by mouth.
- Take VOYDEYA with or without food.
- Take your doses of VOYDEYA around the same time every day.
- If you forget to take your scheduled dose, take it as soon as you remember. If it is within 3 hours of your next dose, skip the missed dose and take your next scheduled dose at your regularly scheduled time. Do not take 2 doses of VOYDEYA at the same time.
- If you take too much VOYDEYA, call your healthcare provider or go to the nearest emergency room right away.
-
If you stop taking VOYDEYA, your healthcare provider will need to monitor you closely for at least 2 weeks after the last dose. Stopping treatment with VOYDEYA may cause a breakdown of red blood cells due to PNH.
Symptoms or problems that can happen due to breakdown of red blood cells include:- decreased hemoglobin level in your blood
- tiredness
What are the possible side effects of VOYDEYA? VOYDEYA may cause side effects, including: - See "What is the most important information I should know about VOYDEYA?"
- Increased liver enzyme levels. Your healthcare provider will do blood tests to check your liver enzyme levels before and during treatment with VOYDEYA. Your healthcare provider may temporarily or permanently stop treatment with VOYDEYA if you develop increased liver enzyme levels.
- Increased cholesterol. VOYDEYA may increase your cholesterol. Your healthcare provider will do blood tests to check your cholesterol during treatment with VOYDEYA.
The most common side effect of VOYDEYA is headache. Tell your healthcare provider about any side effect that bothers you or that does not go away. These are not all of the possible side effects of VOYDEYA. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. How should I store VOYDEYA? - Store VOYDEYA in the original container between 68°F and 77°F (20°C and 25°C).
- Keep VOYDEYA and all medicines out of the reach of children.
General information about the safe and effective use of VOYDEYA. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use VOYDEYA for a condition for which it was not prescribed. Do not give VOYDEYA to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about VOYDEYA that is written for health professionals. What are the ingredients in VOYDEYA? Active ingredient: danicopan Inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, hypromellose acetate succinate, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and sodium lauryl sulfate. The film coatings contain the following inactive ingredients: polyethylene glycol, polyvinyl alcohol, talc, and titanium dioxide. Manufactured for: Alexion Pharmaceuticals, Inc., 121 Seaport Boulevard, Boston, MA 02210 USA
VOYDEYA is a trademark of Alexion Pharmaceuticals, Inc.
© 2024 Alexion Pharmaceuticals, Inc
For more information, go to www.VOYDEYA.com or call 1-888-765-4747. -
VOYDEYA increases your chance of getting serious infections caused by encapsulated bacteria, including Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae type B. These serious infections may quickly become life-threatening and cause death if not recognized and treated early.
-
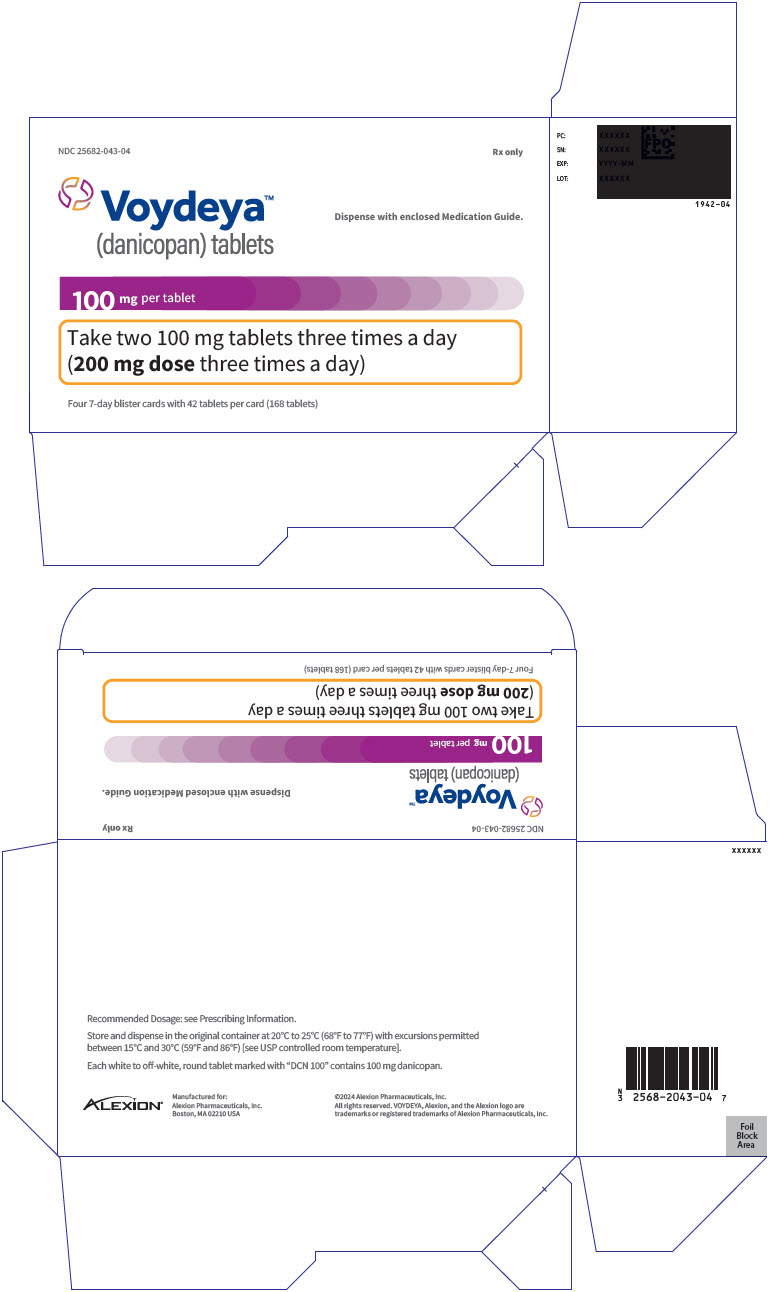
PRINCIPAL DISPLAY PANEL - 100 mg Tablet Blister Pack Carton - 043-04
NDC: 25682-043-04
Rx onlyVoydeya™
(danicopan) tabletsDispense with enclosed Medication Guide.
100 mg per tablet
Take two 100 mg tablets three times a day
(200 mg dose three times a day)Four 7-day blister cards with 42 tablets per card (168 tablets)
-
PRINCIPAL DISPLAY PANEL - 100 mg Tablet Bottle Carton - 043-92
NDC: 25682-043-92
Rx onlyVoydeya™
(danicopan) tabletsDispense with enclosed Medication Guide.
100 mg
180 tablets
90 x 100 mg tablets per bottle
-
PRINCIPAL DISPLAY PANEL - Kit Carton - 049
NDC: 25682-049-04
Rx onlyVoydeya™
(danicopan) tabletsDispense with enclosed Medication Guide.
50 mg per tablet
and
100 mg per tabletTake one 100 mg tablet and one 50 mg tablet
three times a day
(150 mg dose three times a day)Four 7-day blister cards with 42 tablets per card (168 tablets)
21 x 50 mg per tablet
21 x 100 mg per tablet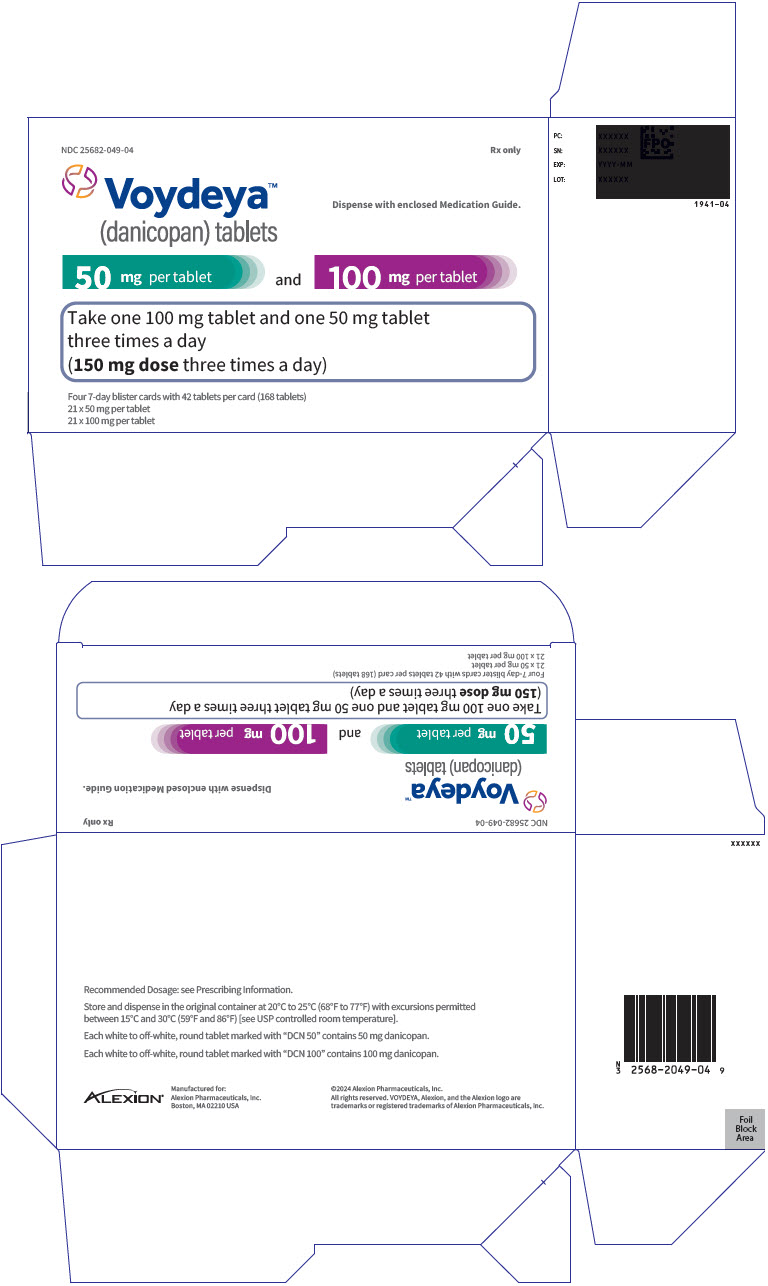
-
PRINCIPAL DISPLAY PANEL - Kit Carton - 046
NDC: 25682-046-92
Rx onlyVoydeya™
(danicopan) tabletsDispense with enclosed Medication Guide.
50 mg
and
100 mgTake one 100 mg tablet and one 50 mg tablet
three times a day
(150 mg dose three times a day)180 tablets
90 x 50 mg tablets per bottle
90 x 100 mg tablets per bottle
-
INGREDIENTS AND APPEARANCE
VOYDEYA
danicopan tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 25682-043 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Danicopan (UNII: JM8C1SFX0U) (Danicopan - UNII:JM8C1SFX0U) Danicopan 100 mg Product Characteristics Color WHITE (white to off-white) Score no score Shape ROUND Size 10mm Flavor Imprint Code DCN;100 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 25682-043-04 4 in 1 CARTON 03/29/2024 1 NDC: 25682-043-42 42 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC: 25682-043-92 2 in 1 CARTON 03/29/2024 2 90 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA218037 03/29/2024 VOYDEYA
danicopan kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 25682-049 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 25682-049-04 4 in 1 CARTON 03/29/2024 1 NDC: 25682-049-42 1 in 1 BLISTER PACK Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BLISTER PACK 21 Part 2 1 BLISTER PACK 21 Part 1 of 2 VOYDEYA
danicopan tablet, film coatedProduct Information Item Code (Source) NDC: 25682-040 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Danicopan (UNII: JM8C1SFX0U) (Danicopan - UNII:JM8C1SFX0U) Danicopan 50 mg Product Characteristics Color WHITE (white to off-white) Score no score Shape ROUND Size 8mm Flavor Imprint Code DCN;50 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 25682-040-21 21 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA218037 03/29/2024 Part 2 of 2 VOYDEYA
danicopan tablet, film coatedProduct Information Item Code (Source) NDC: 25682-043 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Danicopan (UNII: JM8C1SFX0U) (Danicopan - UNII:JM8C1SFX0U) Danicopan 100 mg Product Characteristics Color WHITE (white to off-white) Score no score Shape ROUND Size 10mm Flavor Imprint Code DCN;100 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 25682-043-21 21 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA218037 03/29/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA218037 03/29/2024 VOYDEYA
danicopan kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 25682-046 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 25682-046-92 1 in 1 CARTON 03/29/2024 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 90 Part 2 1 BOTTLE 90 Part 1 of 2 VOYDEYA
danicopan tablet, film coatedProduct Information Item Code (Source) NDC: 25682-040 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Danicopan (UNII: JM8C1SFX0U) (Danicopan - UNII:JM8C1SFX0U) Danicopan 50 mg Product Characteristics Color WHITE (white to off-white) Score no score Shape ROUND Size 8mm Flavor Imprint Code DCN;50 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 25682-040-90 90 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA218037 03/29/2024 Part 2 of 2 VOYDEYA
danicopan tablet, film coatedProduct Information Item Code (Source) NDC: 25682-043 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Danicopan (UNII: JM8C1SFX0U) (Danicopan - UNII:JM8C1SFX0U) Danicopan 100 mg Product Characteristics Color WHITE (white to off-white) Score no score Shape ROUND Size 10mm Flavor Imprint Code DCN;100 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 25682-043-90 90 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA218037 03/29/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA218037 03/29/2024 Labeler - Alexion Pharmaceuticals Inc. (789359510)
Trademark Results [Voydeya]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VOYDEYA 98426805 not registered Live/Pending |
Alexion Pharmaceuticals, Inc. 2024-02-29 |
 VOYDEYA 97515689 not registered Live/Pending |
Alexion Pharmaceuticals, Inc. 2022-07-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.