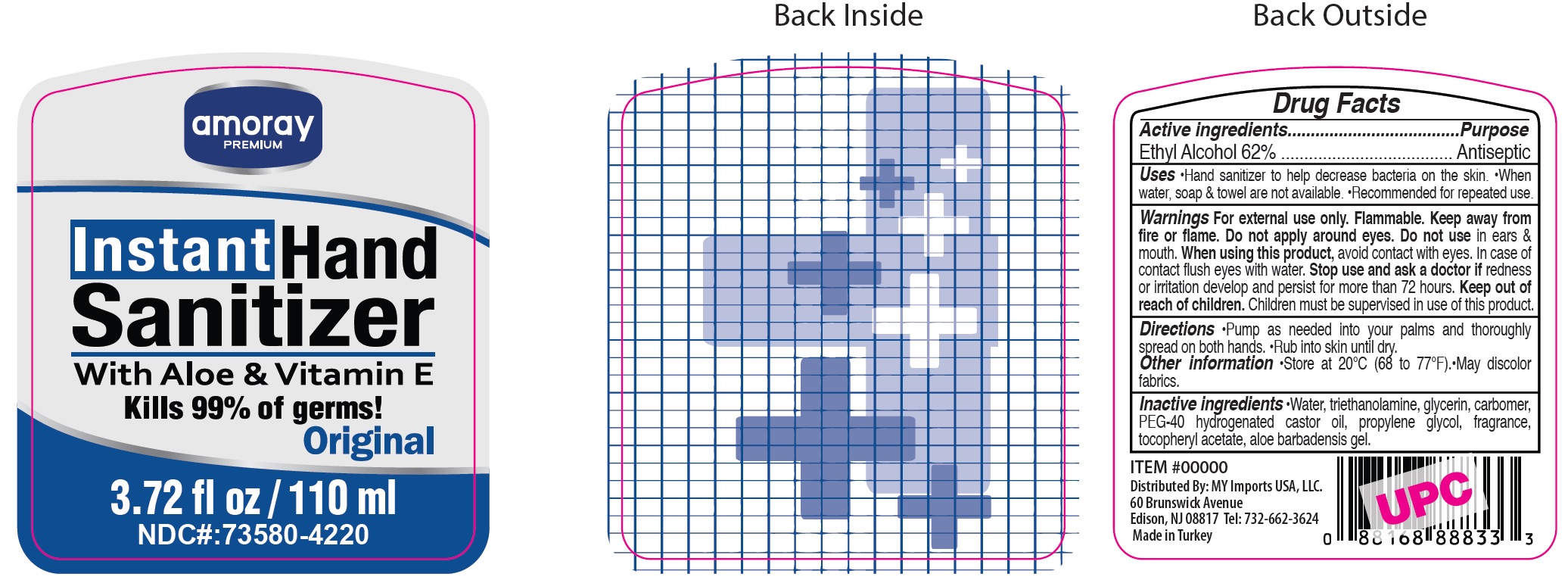
Amoray Premium Hand Sanitizer
Amoray Premium Hand Sanitizer by
Drug Labeling and Warnings
Amoray Premium Hand Sanitizer by is a Otc medication manufactured, distributed, or labeled by My Imports USA LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
AMORAY PREMIUM HAND SANITIZER- alcohol gel
My Imports USA LLC
----------
Amoray Premium Hand Sanitizer
Uses
Hand sanitizer to help decrease bacteria on the skin. When water, soap & towel are not available. Recommended for repeated use.
Directions
Pump as needed into your palms and thoroughly spread on both hands. Rub into skin until dry.
| AMORAY PREMIUM HAND SANITIZER
alcohol gel |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - My Imports USA LLC (195767988) |
Revised: 11/2024
Document Id: 27645be9-3578-96ee-e063-6394a90a743a
Set id: abab3301-990c-41cc-e053-2a95a90a2980
Version: 3
Effective Time: 20241120
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.