Glacier Blue Antibacterial Foaming Skin Cleanser
Glacier Blue Antibacterial Foaming Skin Cleanser by
Drug Labeling and Warnings
Glacier Blue Antibacterial Foaming Skin Cleanser by is a Otc medication manufactured, distributed, or labeled by Lawson Products, Inc, Betco Corporation, Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
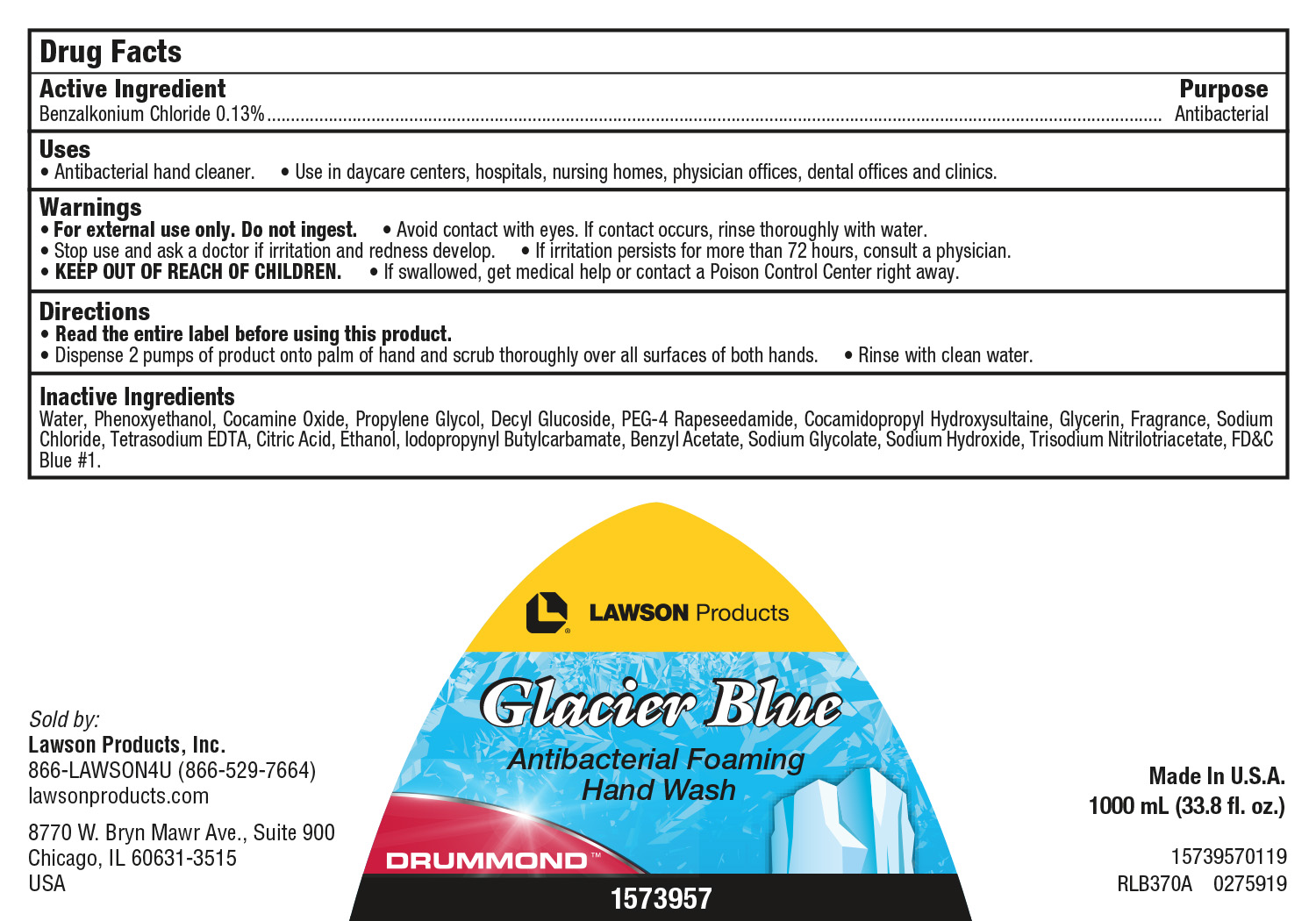
GLACIER BLUE ANTIBACTERIAL FOAMING SKIN CLEANSER- benzalkonium chloride soap
Lawson Products, Inc
----------
Glacier Blue Antibacterial Foaming Skin Cleanser
Warnings
- For external use only. Do not ingest.
- Avoid contact with eyes. If contact occurs, rinse thoroughly with water.
- Stop use and ask a doctor if irritation and redness develop.
- If irritation persists for more than 72 hours, consult a physician.
- KEEP OUT OF REACH OF CHILDREN.
- If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- ?Read the entire label before using this product.
- ?Dispense 2 pumps of product onto palm of hand and scrub thoroughly over all surfaces of both hands.
- Rinse with clean water.
Inactive Ingredients
Water, Phenoxyethanol, Cocamine Oxide, Propylene Glycol, Decyl Glucoside, PEG-4 Rapeseedamide, Cocamidopropyl Hydroxysultaine, Glycerin, Fragrance, Sodium Chloride, Tetrasodium EDTA, Citric Acid, Ethanol, Iodopropynyl Butylcarbamate, Benzyl Acetate, Sodium Glycolate, Sodium Hydroxide, Trisodium Nitrilotriacetate, Diethyl Phthalate, FD&C Blue #1.
| GLACIER BLUE ANTIBACTERIAL FOAMING SKIN CLEANSER
benzalkonium chloride soap |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Lawson Products, Inc (005438890) |
| Registrant - Betco Corporation, Ltd (024492831) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Betco Corporation, Ltd | 024492831 | manufacture(62428-759) , label(62428-759) | |
Revised: 10/2025
Document Id: 4199c676-618a-4302-e063-6294a90ad563
Set id: abacbd35-df47-77ff-e053-2a95a90a6d0b
Version: 3
Effective Time: 20251020
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
 bag-1000ml 759-29
bag-1000ml 759-29