ORIBE SERENE SCALP ANTI DANDRUFF lotion
Oribe Serene Scalp Anti Dandruff by
Drug Labeling and Warnings
Oribe Serene Scalp Anti Dandruff by is a Otc medication manufactured, distributed, or labeled by Oribe Hair Care LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
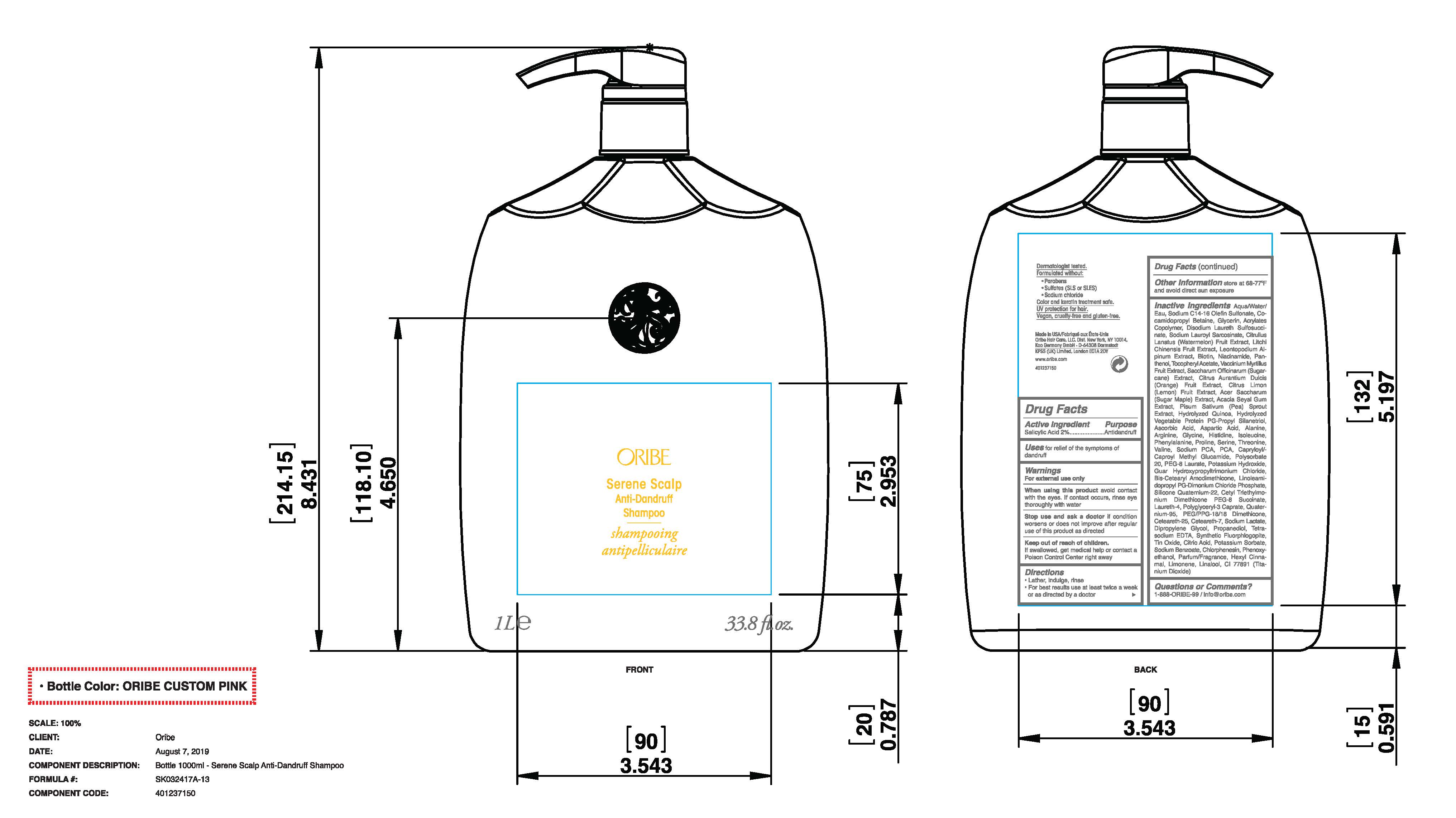
- Active Ingredients
- Uses
- Purpose
- Warnings
- Keep out of reach of children.
- Directions
- Other Information
-
Inactive Ingredients
Aqua/Water/Eau, Sodium C14-16 Olefin Sulfonate, Cocamidopropyl Betaine, Glycerin, Acrylates Copolymer, Disodium Laureth Sulfosuccinate, Sodium Lauroyl Sarcosinate, Citrullus Lanatus (Watermelon) Fruit Extract, Litchi Chinensis Fruit Extract, Leontopodium Alpinum Extract, Biotin, Niacinamide, Panthenol, Tocopheryl Acetate, Vaccinium Myrtillus Fruit Extract, Saccharum Officinarum (Sugarcane) Extract, Citrus Aurantium Dulcis (Orange) Fruit Extract, Citrus Limon (Lemon) Fruit Extract, Acer Saccharum (Sugar Maple) Extract, Acacia Seyal Gum Extract, Pisum Sativum (Pea) Sprout Extract, Hydrolyzed Quinoa, Hydrolyzed Vegetable Protein PG-Propyl Silanetriol, Ascorbic Acid, Aspartic Acid, Alanine, Arginine, Glycine, Histidine, Isoleucine, Phenylalanine, Proline, Serine, Threonine, Valine, Sodium PCA, PCA, Capryloyl/Caproyl Methyl Glucamide, Polysorbate 20, PEG-8 Laurate, Potassium Hydroxide, Guar Hydroxypropyltrimonium Chloride, Bis-Cetearyl Amodimethicone, Linoleamidopropyl PG-Dimonium Chloride Phosphate, Silicone Quaternium-22, Cetyl Triethylmonium Dimethicone PEG-8 Succinate, Laureth-4, Polyglyceryl-3 Caprate, Quaternium-95, PEG/PPG-18/18 Dimethicone, Ceteareth-25, Ceteareth-7, Sodium Lactate, Dipropylene Glycol, Propanediol, Tetrasodium EDTA, Synthetic Fluorphlogopite, Tin Oxide, Citric Acid, Potassium Sorbate, Sodium Benzoate, Chlorphenesin, Phenoxyethanol, Parfum/Fragrance, Hexyl Cinnamal, Limonene, Linalool, CI 77891 (Titanium Dioxide)
- Questions or Comments?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
ORIBE SERENE SCALP ANTI DANDRUFF
oribe serene scalp anti dandruff lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 71438-010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 2 g in 100 g Inactive Ingredients Ingredient Name Strength VALINE (UNII: HG18B9YRS7) POLYSORBATE 20 (UNII: 7T1F30V5YH) PEG-8 LAURATE (UNII: 762O8IWA10) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) GUAR HYDROXYPROPYLTRIMONIUM CHLORIDE (1.7 SUBSTITUENTS PER SACCHARIDE) (UNII: B16G315W7A) LAURETH-4 (UNII: 6HQ855798J) QUATERNIUM-91 (UNII: 00J8H295NB) CITRIC ACID ACETATE (UNII: DSO12WL7AU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM BENZOATE (UNII: OJ245FE5EU) PHENOXYETHANOL (UNII: HIE492ZZ3T) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) WATER (UNII: 059QF0KO0R) SODIUM C14-16 OLEFIN SULFONATE (UNII: O9W3D3YF5U) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) DISODIUM LAURETH SULFOSUCCINATE (UNII: D6DH1DTN7E) SODIUM LAUROYL SARCOSINATE (UNII: 632GS99618) CITRULLUS LANATUS VAR. LANATUS WHOLE (UNII: 3J5I6254YO) LITCHI CHINENSIS WHOLE (UNII: 47EV26G96N) LEONTOPODIUM ALPINUM FLOWER (UNII: MWN6IZU3XM) BIOTIN (UNII: 6SO6U10H04) NIACINAMIDE (UNII: 25X51I8RD4) PANTHENOL (UNII: WV9CM0O67Z) TOCOPHEROL (UNII: R0ZB2556P8) VACCINIUM MYRTILLUS WHOLE (UNII: EE76EZ4QNZ) SACCHARUM OFFICINARUM WHOLE (UNII: 3Z20C92XNB) ACACIA SENEGAL WHOLE (UNII: QP4QYZ033C) PISUM SATIVUM WHOLE (UNII: J21YE3W98E) ASCORBIC ACID (UNII: PQ6CK8PD0R) ASPARTIC ACID (UNII: 30KYC7MIAI) ALANINE (UNII: OF5P57N2ZX) ARGININE (UNII: 94ZLA3W45F) GLYCINE (UNII: TE7660XO1C) HISTIDINE (UNII: 4QD397987E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71438-010-20 1 in 1 CARTON 10/05/2022 1 1000 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M032 10/05/2022 Labeler - Oribe Hair Care LLC (008843347) Registrant - Oribe Hair Care LLC (008843347)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.