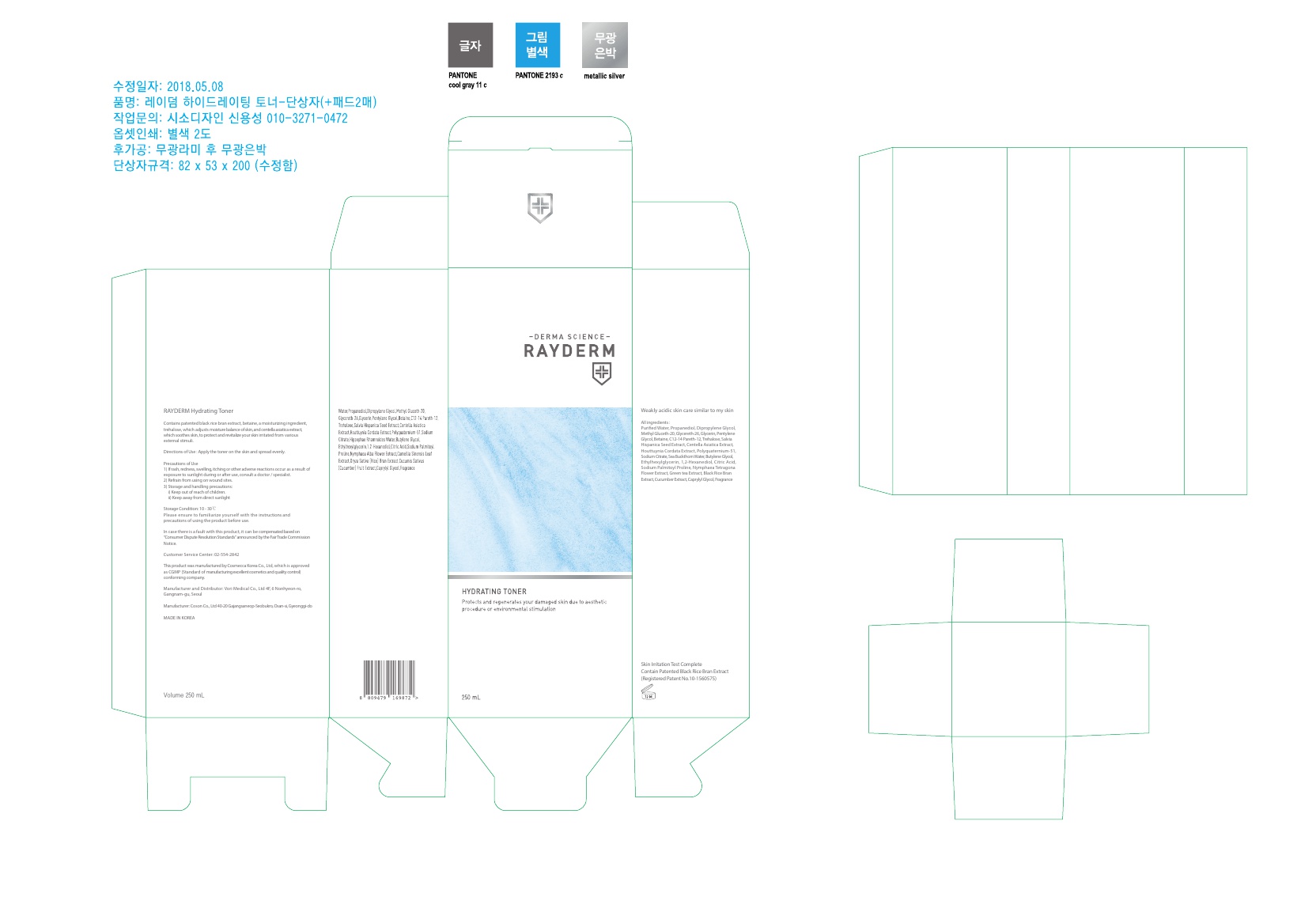
RAYDERM HYDRATING TONER- glycerin toner liquid
Rayderm Hydrating Toner by
Drug Labeling and Warnings
Rayderm Hydrating Toner by is a Otc medication manufactured, distributed, or labeled by VORY MEDICAL. Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
Purified Water, Propanediol, Dipropylene, Methyl Gluceth-20, Glycereth-26, Glycerin, Pentylene Glycol, Betaine, C12-14 Pareth-12, Trehalose, Salvia Hispanica Seed Extract, Centella Asiatica Extract, Houttuynia Cordata Extract, Polyquaternium-51, Sodium Citrate, Sea Buckthorn Water, Butylene Glycol, Ethylhexylglycerin, 1,2-Hexanediol, Citric Acid, Sodium Palmitoyl Proline, Nymphaea Tetragona Flower Extract, Green tea Extract, Black Rice Bran Extract, Cucumber Extract, Caprylyl Glycol, Fragrance
- PURPOSE
-
WARNINGS
Precautions of Use
1) If rash, redness, swelling, itching or other adverse reactions occur as a result of exposure to sunlight during or after use, consult a doctor / specialist.
2) Refrain from using on wound sites.
3) Storage and handling precautions:
i) Keep out of reach of children.
ii) Keep away from direct sunlight - KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
RAYDERM HYDRATING TONER
glycerin toner liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 72543-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength glycerin (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) glycerin 6.25 g in 250 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) PROPANEDIOL (UNII: 5965N8W85T) DIPROPYLENE GLYCOL (UNII: E107L85C40) METHYL GLUCETH-20 (UNII: J3QD0LD11P) GLYCERETH-26 (UNII: NNE56F2N14) PENTYLENE GLYCOL (UNII: 50C1307PZG) BETAINE (UNII: 3SCV180C9W) C12-14 PARETH-12 (UNII: M0LJS773XW) TREHALOSE (UNII: B8WCK70T7I) SALVIA HISPANICA SEED (UNII: NU0OLX06F8) CENTELLA ASIATICA (UNII: 7M867G6T1U) POLYQUATERNIUM-51 (2-METHACRYLOYLOXYETHYL PHOSPHORYLCHOLINE/N-BUTYL METHACRYLATE; 3:7) (UNII: EL9825H96J) SODIUM CITRATE (UNII: 1Q73Q2JULR) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) SODIUM PALMITOYL PROLINE (UNII: 64L053FRFO) NYMPHAEA ALBA FLOWER (UNII: 40KQ7Q535O) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72543-004-01 250 mL in 1 PACKAGE; Type 0: Not a Combination Product 10/11/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/11/2018 Labeler - VORY MEDICAL. Inc (694721104) Registrant - VORY MEDICAL. Inc (694721104) Establishment Name Address ID/FEI Business Operations VORY MEDICAL. Inc 694721104 manufacture(72543-004)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.