TRANZGEL- echinacea angustifolia, echinacea purpurea, aconitum napellus, arnica montana, calendula officianalis, hamamelis virginiana, belladonna, bellis perennis, chamomillia, millefolium, hypericum perforatum, symphytum officinale, colchicinum gel
TRANZGEL by
Drug Labeling and Warnings
TRANZGEL by is a Homeopathic medication manufactured, distributed, or labeled by Gensco Laboratories, LLC, Formulated Solutions. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
ACTIVE INGREDIENT
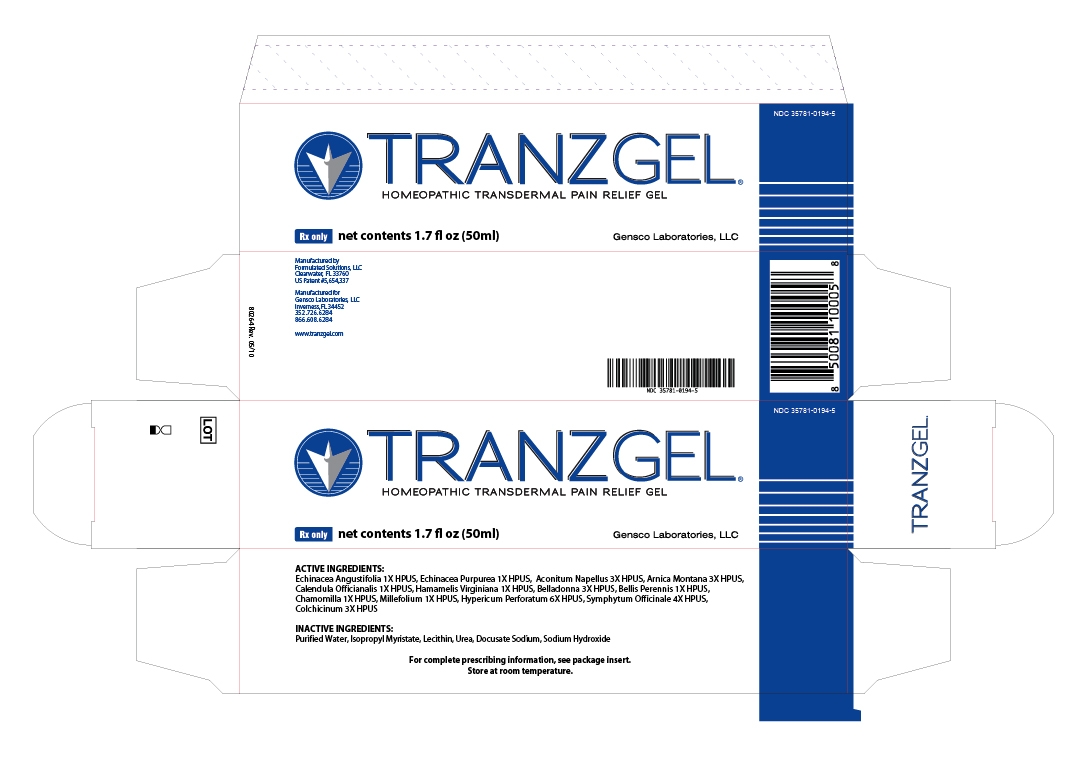
ACTIVE INGREDIENTS:
ECHINACEA ANGUSTIFOLIA 1X HPUS, ECHINACEA PUPUREA 1X HPUS, ACONITUM NAPELLUS 3X HPUS, ARNICA MONTANA 3X HPUS, CALENDULA OFFICIANALIS 1X HPUS, HAMAMELIS VIRGINIANA 1X HPUS, BELLADONNA 3X HPUS, BELLIS PERENNIS 1X HPUS, CHAMOMILLA 1X HPUS, MILLEFOLIUM 1X HPUS, HYPERICUM PERFORATUM 6X HPUS, SYMPHYTUM OFFICINALE 4X HPUS, COLCHICINUM 3X HPUS
- INACTIVE INGREDIENT
-
PRINCIPAL DISPLAY PANEL
NDC: 35781-0194-5
TRANZGEL
HOMEOPATHIC TRANSDERMAL PAIN RELIEF GEL
RX ONLY NET CONTENTS 1.7 FL OZ (50 ML)
GENSCO LABORATORIES, LLC
FOR COMPLETE PRESCRIBING INFORMATION, SEE PACKAGE INSERT. STORE AT ROOM TEMPERATURE.
MANUFACTURED BY FORMULATED SOLUTIONS
CLEARWATER, FL 33760
US PATENT #5,654,337
MANUFACTURED FOR GENSCO LABORATORIES, LLC
INVERNESS, FL 34452
352.726.6284
866.608.6284
WWW.TRANZGEL.COM
- PRINCIPAL DISPLAY PANEL
- SPL UNCLASSIFIED SECTION
-
ACTIVE INGREDIENT
ACTIVE INGREDIENTS:
ECHINACEA ANGUSTIFOLIA 1X HPUS, ECHINACEA PUPUREA 1X HPUS, ACONITUM NAPELLUS 3X HPUS, ARNICA MONTANA 3X HPUS, CALENDULA OFFICIANALIS 1X HPUS, HAMAMELIS VIRGINIANA 1X HPUS, BELLADONNA 3X HPUS, BELLIS PERENNIS 1X HPUS, CHAMOMILLA 1X HPUS, MILLEFOLIUM 1X HPUS, HYPERICUM PERFORATUM 6X HPUS, SYMPHYTUM OFFICINALE 4X HPUS, COLCHICINUM 3X HPUS - INACTIVE INGREDIENT
- SPL UNCLASSIFIED SECTION
- DESCRIPTION
-
ACTIVE INGREDIENT
ACTIVE INGREDIENTS:
50 PARTS EACH: ECHINACEA ANGUSTIFOLIA 1X HPUS, ECHINACEA PUPUREA 1X HPUS, 30 PARTS: ACONITUM NAPELLUS 3X HPUS, 15 PARTS EACH: ARNICA MONTANA 3X HPUS, CALENDULA OFFICIANALIS 1X HPUS, HAMAMELIS VIRGINIANA 1X HPUS, 10 PARTS: BELLADONNA 3X HPUS, 5 PARTS EACH: BELLIS PERENNIS 1X HPUS, CHAMOMILLA 1X HPUS, 3 PARTS MILLEFOLIUM 1X HPUS, 1 PART EACH: HYPERICUM PERFORATUM 6X HPUS, SYMPHYTUM OFFICINALE 4X HPUS, .01 PART COLCHICINUM 3X HPUS - INACTIVE INGREDIENT
- Physical Information
-
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGY
According to traditional homeopathic literature and Material Medica, the following ingredients provide the following attributes:
INGREDIENTS COMMON NAME INDICATIONS
Arnica Montana Mountain arnica Stimulates healing of injured tissues wounds, contusions, hematomas, neuralgia myalgia, analgesia
Calendula Officianalis Calendula Inflammation
Hamamelis Virginiana Witch-hazel Analgesic
Millefolium Yarrow Hematomas
Belladonna Banewort Inflammation locally
Aconitum Napellus Monk's hood Neuralgia, rheumatism hemostasis, analgeisa
Chamomilla Chamomile Inflammation, nausea
Colchicinum Colchicine Inflammation and gout
Symphytum Officinale Comfrey Neuropathy, contusions, tendonitis, arthritis
Bellis Perennis Daisy brusing, edema, arthralgia
Echinacea Angustifolia Narrow leaf cone flower Inflammation, myalgia
Echinacia purpurea purple cone flower Inflammation, myalgia
Hypericum perforatum st. john's wort neuropathic pains
-
INDICATIONS & USAGE
INDICATIONS AND USAGE
The drug is indicated for the relief of symptoms, including pain and inflammation associated with arthritis or trauma (such as sprains, strains, dislocations, repetitive/overuse injuries, traumatic, edema, post surgical edema, hematoma, general swelling of joints and soft tissues) to areas such as hand, wrist elbow, shoulder, neck, back, knees, ankles, feet and toes.
- CONTRAINDICATIONS
-
WARNINGS AND PRECAUTIONS
WARNINGS AND PRECAUTIONS
For external use only. direct patient not to ingest TRANZEL and to avoid contact with they eyes, mucous membranes, wounds, and damaged skin. If a rash develops, patient should discontinue use until rash clears. After the disappearance of rash, patient can try TRANZGEL again on a test area and monitor the site for additional results. If no rash or redness results, then patient can resume use. However, if the rash persists or redevelops, use should be discontinued.
Direct the patient to keep this product out of reach of children and seek medical help or contact a Poison Control Center immediately if swallowed.
In the homeopathic concentrations used to make TRANZGEL, there are no known or expected interactions with other drugs or laboratory tests. In addition, the homeopathic concentrations used are below any levels with known or suspected toxicities.
- OVERDOSAGE
- ADVERSE REACTIONS
- DOSAGE & ADMINISTRATION
-
HOW SUPPLIED
Package size: 1.7 fl oz (50 ml) airless pump dispensing bottle
US PATENT 5,654,337
NDC: 35781-0194-5
- INFORMATION FOR PATIENTS
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TRANZGEL
echinacea angustifolia, echinacea purpurea, aconitum napellus, arnica montana, calendula officianalis, hamamelis virginiana, belladonna, bellis perennis, chamomillia, millefolium, hypericum perforatum, symphytum officinale, colchicinum gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 35781-0194 Route of Administration TRANSDERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ECHINACEA ANGUSTIFOLIA (UNII: VB06AV5US8) (ECHINACEA ANGUSTIFOLIA - UNII:VB06AV5US8) ECHINACEA ANGUSTIFOLIA 1 [hp_X] in 50 mL ECHINACEA PURPUREA (UNII: QI7G114Y98) (ECHINACEA PURPUREA - UNII:QI7G114Y98) ECHINACEA PURPUREA 1 [hp_X] in 50 mL ACONITUM NAPELLUS (UNII: U0NQ8555JD) (ACONITUM NAPELLUS - UNII:U0NQ8555JD) ACONITUM NAPELLUS 3 [hp_X] in 50 mL ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 3 [hp_X] in 50 mL CALENDULA OFFICINALIS FLOWERING TOP (UNII: 18E7415PXQ) (CALENDULA OFFICINALIS FLOWERING TOP - UNII:18E7415PXQ) CALENDULA OFFICINALIS FLOWERING TOP 1 [hp_X] in 50 mL HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK (UNII: T7S323PKJS) (HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK - UNII:T7S323PKJS) HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK 1 [hp_X] in 50 mL ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 3 [hp_X] in 50 mL BELLIS PERENNIS (UNII: 2HU33I03UY) (BELLIS PERENNIS - UNII:2HU33I03UY) BELLIS PERENNIS 1 [hp_X] in 50 mL CHAMOMILE (UNII: FGL3685T2X) (CHAMOMILE - UNII:FGL3685T2X) CHAMOMILE 1 [hp_X] in 50 mL ACHILLEA MILLEFOLIUM (UNII: 2FXJ6SW4PK) (ACHILLEA MILLEFOLIUM - UNII:2FXJ6SW4PK) ACHILLEA MILLEFOLIUM 1 [hp_X] in 50 mL HYPERICUM OIL (UNII: OZU2FC70HY) (HYPERICUM OIL - UNII:OZU2FC70HY) HYPERICUM OIL 6 [hp_X] in 50 mL COMFREY ROOT (UNII: M9VVZ08EKQ) (COMFREY ROOT - UNII:M9VVZ08EKQ) COMFREY ROOT 4 [hp_X] in 50 mL COLCHICINE (UNII: SML2Y3J35T) (COLCHICINE - UNII:SML2Y3J35T) COLCHICINE 3 [hp_X] in 50 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) UREA (UNII: 8W8T17847W) DOCUSATE SODIUM (UNII: F05Q2T2JA0) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 35781-0194-5 1 in 1 CARTON 1 50 mL in 1 BOTTLE, DISPENSING Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 07/01/2010 Labeler - Gensco Laboratories, LLC (831042325) Establishment Name Address ID/FEI Business Operations Formulated Solutions 143266687 manufacture
Trademark Results [TRANZGEL]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TRANZGEL 77741930 4168267 Live/Registered |
GENSCO LABORATORIES, LLC 2009-05-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.