Allergy Relief by L.N.K. International, Inc. / LNK International, Inc. Quality Plus 44-729
Allergy Relief by
Drug Labeling and Warnings
Allergy Relief by is a Otc medication manufactured, distributed, or labeled by L.N.K. International, Inc., LNK International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
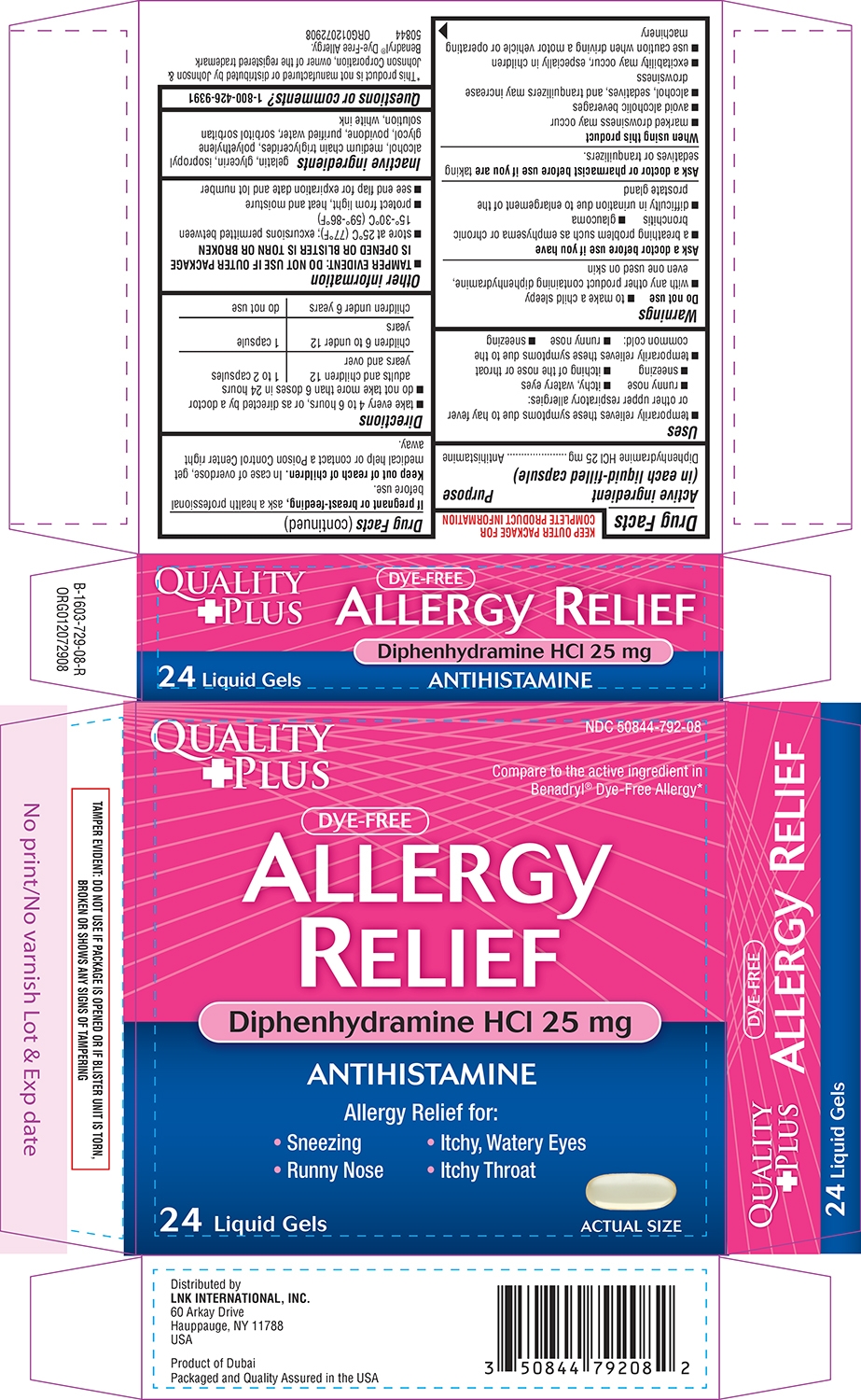
ALLERGY RELIEF- diphenhydramine hcl capsule, liquid filled
L.N.K. International, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Quality Plus 44-729
Uses
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- itchy, watery eyes
- sneezing
- itching of the nose or throat
- temporarily relieves these symptoms due to the common cold:
- runny nose
- sneezing
Warnings
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
Directions
- take every 4 to 6 hours, or as directed by a doctor
- do not take more than 6 doses in 24 hours
| adults and children 12 years and over | 1 to 2 capsules |
| children 6 to under 12 years | 1 capsule |
| children under 6 years | do not use |
Other information
- TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
- protect from light, heat and moisture
- see end flap for expiration date and lot number
Inactive ingredients
gelatin, glycerin, isopropyl alcohol, medium chain triglycerides, polyethylene glycol, povidone, purified water, sorbitol sorbitan solution, white ink
Principal display panel
QUALITY
+PLUS
NDC: 50844-792-08
Compare to the active ingredient in
Benadryl® Dye-Free Allergy*
DYE-FREE
ALLERGY
RELIEF
Diphenhydramine HCl 25 mg
ANTIHISTAMINE
Allergy Relief for:
Sneezing Itchy, Watery Eyes
Runny Nose Itchy Throat
24 Liquid Gels
ACTUAL SIZE
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED OR IF BLISTER UNIT IS TORN,
BROKEN OR SHOWS ANY SIGNS OF TAMPERING
*This product is not manufactured or distributed by Johnson &
Johnson Corporation, owner of the registered trademark
Benadryl® Dye-Free Allergy.
50844 ORG012072908
Distributed by
LNK INTERNATIONAL, INC.
60 Arkay Drive
Hauppauge, NY 11788
USA
Product of Dubai
Packaged and Quality Assured in the USA
Quality Plus 44-729
| ALLERGY RELIEF
diphenhydramine hcl capsule, liquid filled |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - L.N.K. International, Inc. (038154464) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867837 | pack(50844-792) | |
Trademark Results [Allergy Relief]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ALLERGY RELIEF 98236984 not registered Live/Pending |
Dmytro Kononenko 2023-10-24 |
 ALLERGY RELIEF 90457167 not registered Live/Pending |
American Textile Company, Inc. 2021-01-10 |
 ALLERGY RELIEF 78838437 3358249 Live/Registered |
Meshbesher Health Corporation 2006-03-16 |
 ALLERGY RELIEF 76619855 3066888 Live/Registered |
AMERICAN TEXTILE COMPANY 2004-11-09 |
 ALLERGY RELIEF 74668018 not registered Dead/Abandoned |
NaturaLife Corporation 1995-05-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.