Astral Organica Hand Sanitizer with CBD by Kabana Skin Care
Astral Organica Hand Sanitizer with CBD by
Drug Labeling and Warnings
Astral Organica Hand Sanitizer with CBD by is a Otc medication manufactured, distributed, or labeled by Kabana Skin Care. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ASTRAL ORGANICA HAND SANITIZER WITH CBD- hand sanitizer gel
Kabana Skin Care
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
DRUG FACTS
Package Insert, not enough space on label for Drug Facts panel.

Astral Organica Hand Santizer + CBD package label - note QR code referencing Drug Facts that is easily accessible via smart devices.
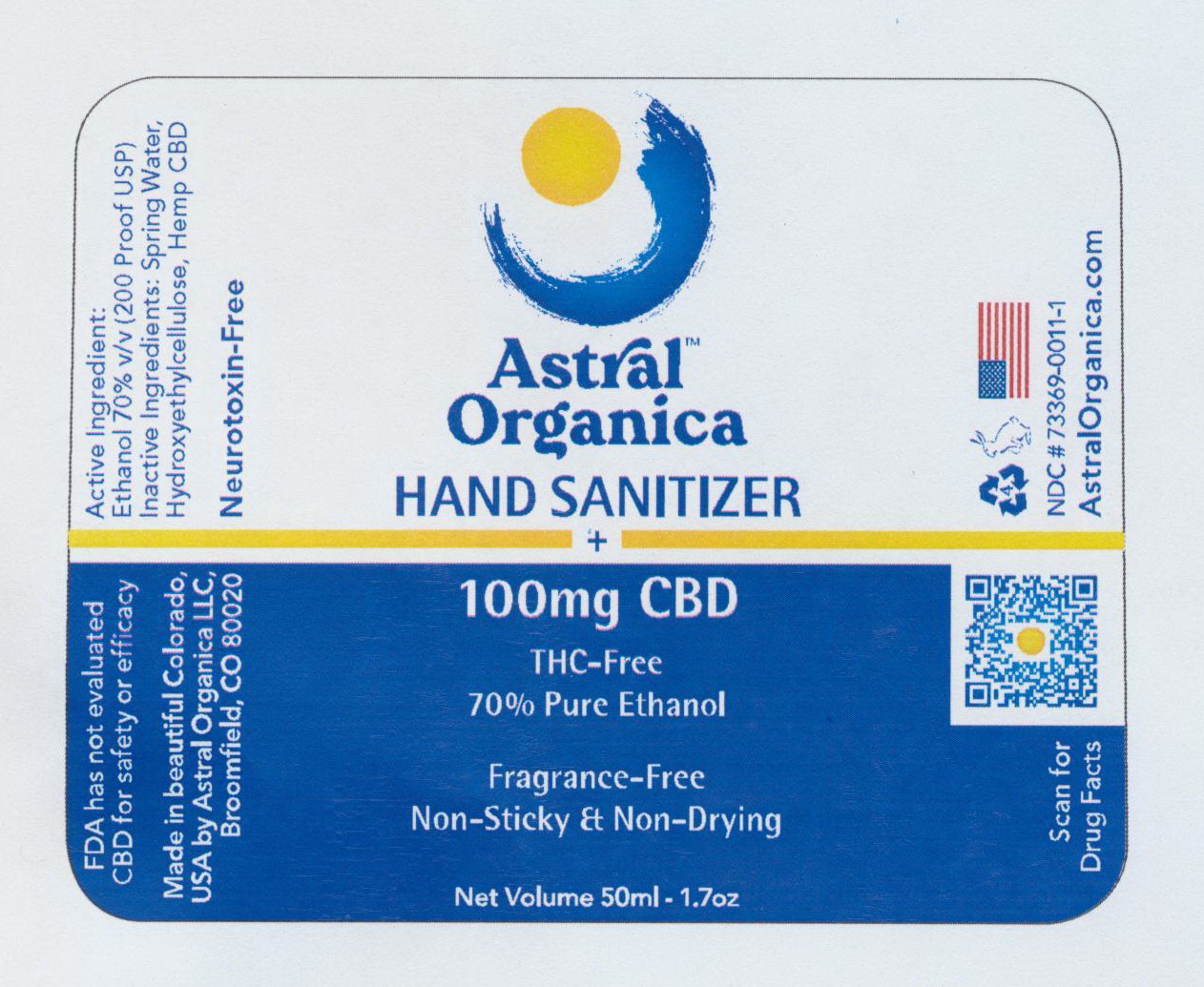
PURPOSE
ANTIMICROBIAL
DISCONTINUE USE IF IRRITATION OR REDNESS DEVELOPS.
IF CONDITION PERSISTS FOR MORE THAN 72 HOURS, CONSULT A DOCTOR.
| ASTRAL ORGANICA HAND SANITIZER WITH CBD
hand sanitizer gel |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Kabana Skin Care (080237112) |
| Registrant - Kabana Skin Care (080237112) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Kabana Skin Care | 080237112 | manufacture(73369-0011) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.