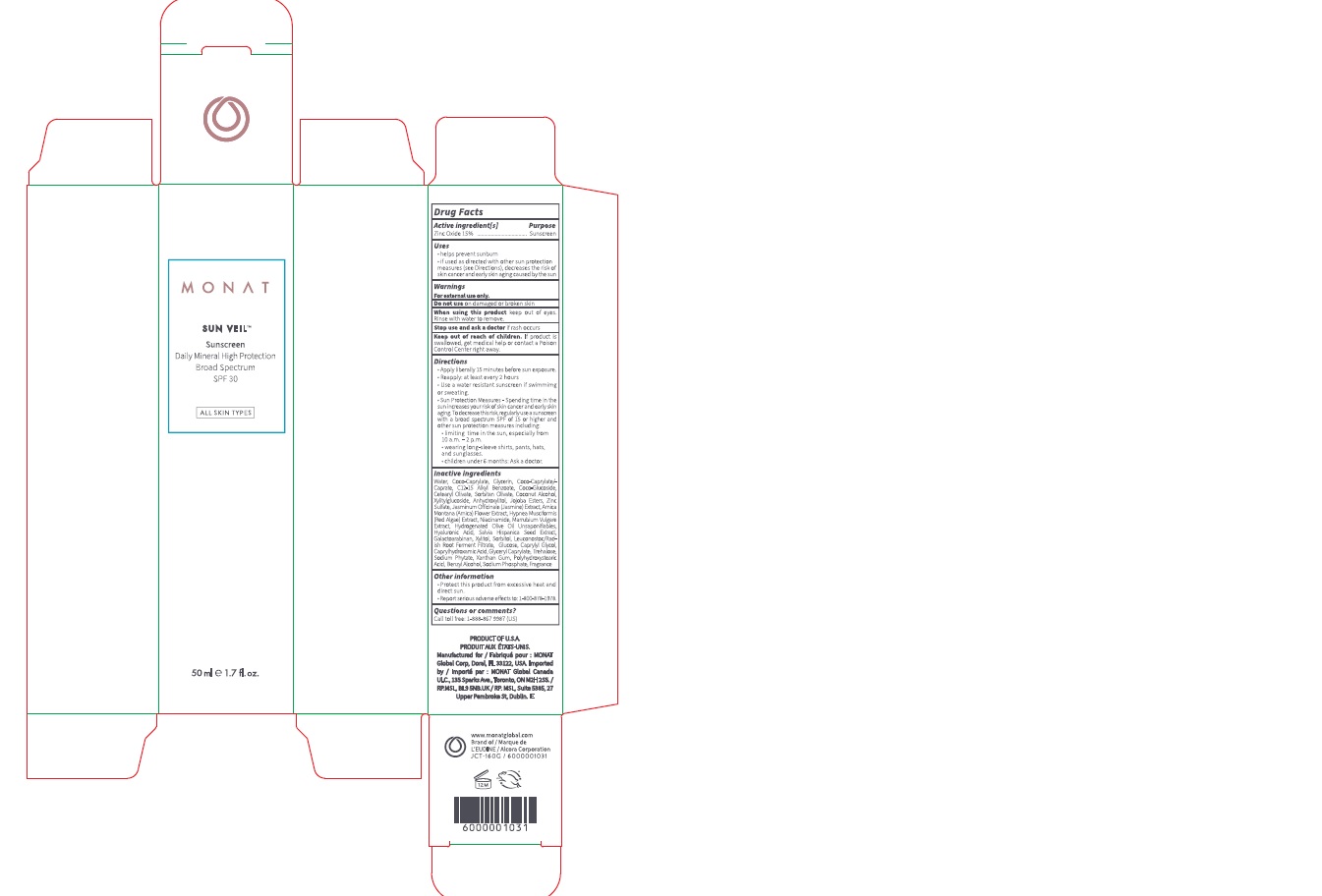
Monat Sun Veil Sunscreen Broad Spectrum SPF 30
Monat Sun Veil Sunscreen Broad Spectrum SPF 30 by
Drug Labeling and Warnings
Monat Sun Veil Sunscreen Broad Spectrum SPF 30 by is a Otc medication manufactured, distributed, or labeled by Monat Global Corp.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
MONAT SUN VEIL SUNSCREEN BROAD SPECTRUM SPF 30- zinc oxide lotion
Monat Global Corp.
----------
Monat Sun Veil Sunscreen Broad Spectrum SPF 30
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Directions
- Apply liberally 15 minutes before sun exposure.
- Reapply: at least every 2 hours
- Use a water resistant sunscreen if swimming or sweating.
- Sun Protection Measures - Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limiting time in the sun, especially from 10 a.m. - 2 p.m.
- wearing long-sleeve shirts, pants, hats, and sunglasses.
- children under 6 months: Ask a doctor.
Inactive ingredients
Water, Coco-Caprylate, Glycerin, Coco-Caprylate/Caprate, C12-15 Alkyl Benzoate, Coco-Glucoside, Cetearyl Olivate, Soibitan Olivate, Coconut Alcohol, Xylitylgucoside, Anhydroxylitol, Jojoba Esters, Zinc Sulfate, Jasminum Officinale (Jasmine) Extract, Arnica Montana (Arnica) Rower Extract, Hypnea Musciformis (Red Algae) Extract, Niacinamide, Marrubium Vulgare Extract, Hydrogenated Olive Oil Unsaponifiables, Hyaluronic Acid, Salvia Hispanica Seed Extract, Galactoarabinan, Xylitol, Sorbitol, Leuconostoc/Rad- ish Root Ferment Filtrate, Glucose, Caprylyl Glycol, CaprylhydroxamicAcid, Glyceryl Caprylate, Trehalose, Sodium Phytate, Xanthan Gum, Polyhydroxystearic Add, Benzyl Alcohol, Sodium Phosphate, Fragrance
Other information
- Protect this product from excessive heat and direct sun.
- Report serious adverse effect to: 1-800-878-1978.
| MONAT SUN VEIL SUNSCREEN BROAD SPECTRUM SPF 30
zinc oxide lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Monat Global Corp. (027036949) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.