Super Cortisone by Skin PS Brands / Westwood Laboratories, Inc. Super Cortisone+
Super Cortisone by
Drug Labeling and Warnings
Super Cortisone by is a Otc medication manufactured, distributed, or labeled by Skin PS Brands, Westwood Laboratories, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
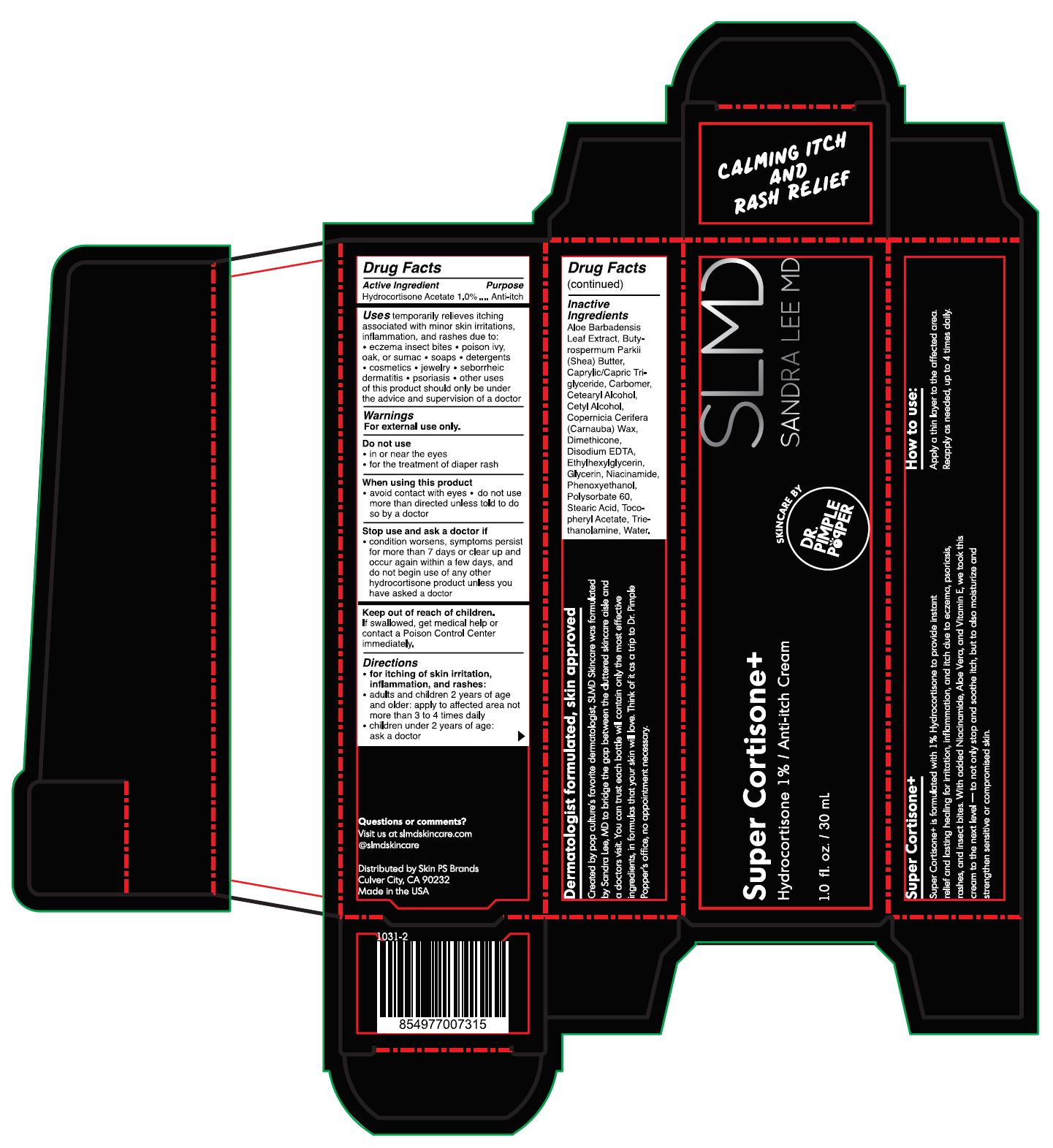
SUPER CORTISONE- hydrocortisone cream
Skin PS Brands
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Super Cortisone+
Uses
temporarily relieves itching associated with minor skin irritations, inflammation, and rashes due to:
- eczema insect bites
- poison ivy, oak or sumac
- soaps
- detergents
- cosmetics
- jewelry
- seborrheic dermatitis
- psoriasis
- other uses of this product should be under the advice and supervision of a doctor.
When using this product
- avoid contact with eyes
- do not use more than directed unless told to do so by a doctor
Stop use and ask a doctor if
- condition worsens, symptoms persist for more than 7 days or clear up and occur again within a few days, and do not begin use of any other hydrocortisone product unless you have asked a doctor
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center immediately.
Directions
- for itching of skin irritation, inflammation, and rashes:
- adults and children 2 years of age and older: apply to affected area not more than 3 to 4 times dailys.
- children under 2 years of age: ask a doctor
Inactive Ingredients
Aloe Barbadensis Leaf Extract, Butyrospermum Parkii (Shea) Butter, Caprylic/Capric Triglyceride, Carbomer, Cetearyl Alcohol, Cetyl Alcohol, Copernicia Cerifera (Carnauba) Wax, Dimethicone, Disodium EDTA, Ethylhexylglycerin, Glycerin, Niacinamide, Phenoxyethanol, Polysorbate 60, Stearic Acid, Tocopherol Acetate, Triethanolamine, Water.
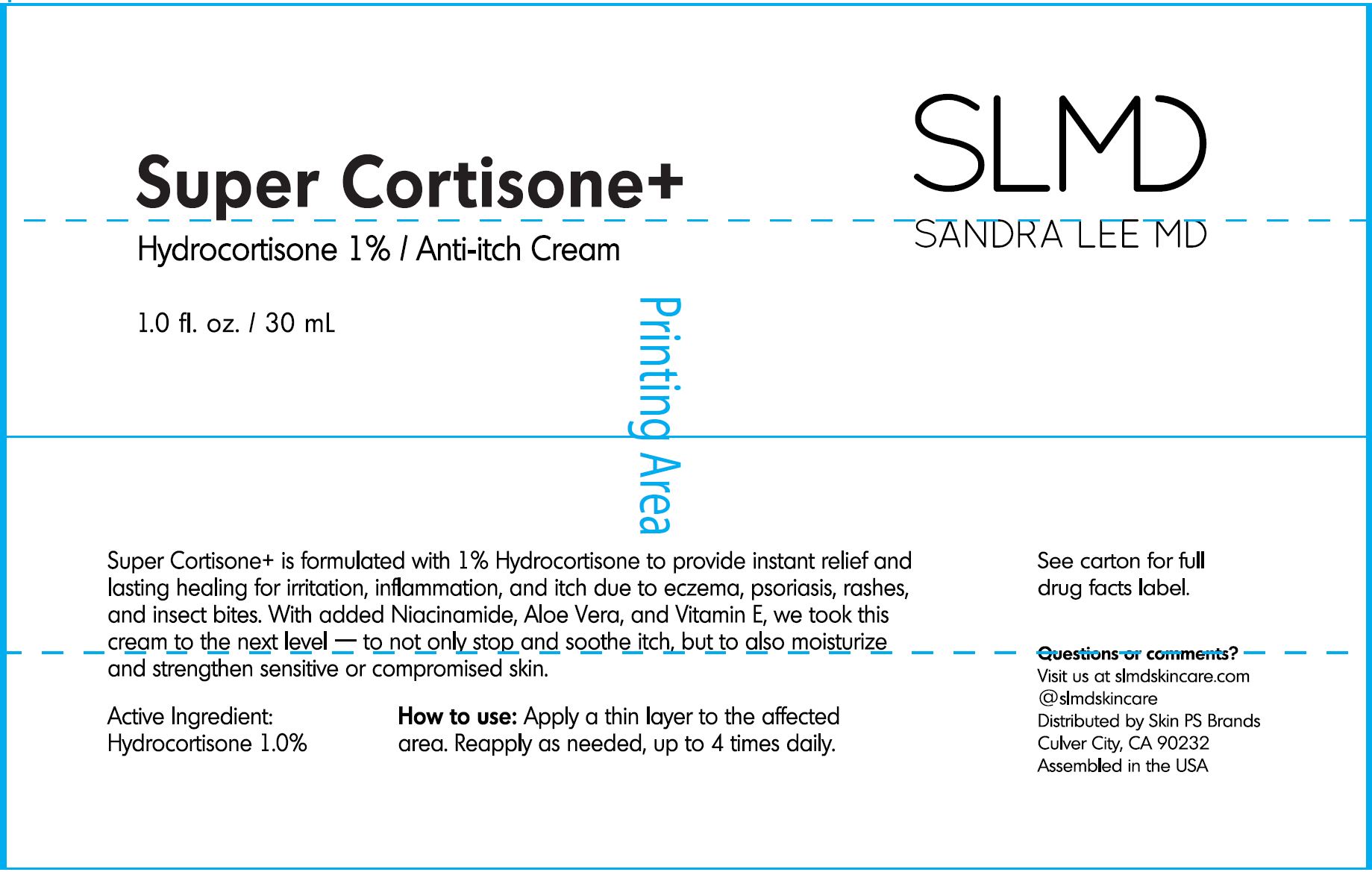
Super Cortisone+
Hydrocortisone 1% / Anti-itch Cream
1.0 fl. oz. / 30 mL
SLMD
SANDRA LEE MD
SKINCARE BY
Dr. Pimple Popper
Super Cortisone+
Super Cortisone+ is formulated with 1% Hydrocortisone to provide instant
relief and lasting healing for irritation, inflammation, and itch due to eczema, psoriasis,
rashes, and insect bites. With added Niacinamide, Aloe Vera, and Vitamin E, we took this
cream to the next level - to not only stop and soothe itch, but to also moisturize and
strengthen sensitive or compromised skin.
How to use:
Apply a thin layer to the affected area.
Reapply as needed, up to 4 times daily.
Dermatologist formulated, skin approved
Created by pop culture's favorite dermatologist, SLMD Skincare was formulated
by Sandra Lee MD to bridge to gap between the cluttered skincare aisles and
a doctors visit. You can trust each bottle will contain only the most effective
ingredients, in formulas that your skin will love. Think of it as a trip to Dr. Pimple
Popper's office, no appointment necessary.
Questions or comments?
Visit us at slmdskincare.com
@slmdskincare
Distributed by Skin PS Brands
Culver City, CA 90232
Made in the USA
Carton Artwork:
Tube Artwork:
| SUPER CORTISONE
hydrocortisone cream |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Skin PS Brands (081085221) |
| Registrant - Skin PS Brands (081085221) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Westwood Laboratories, Inc. | 832280635 | manufacture(73318-1031) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.