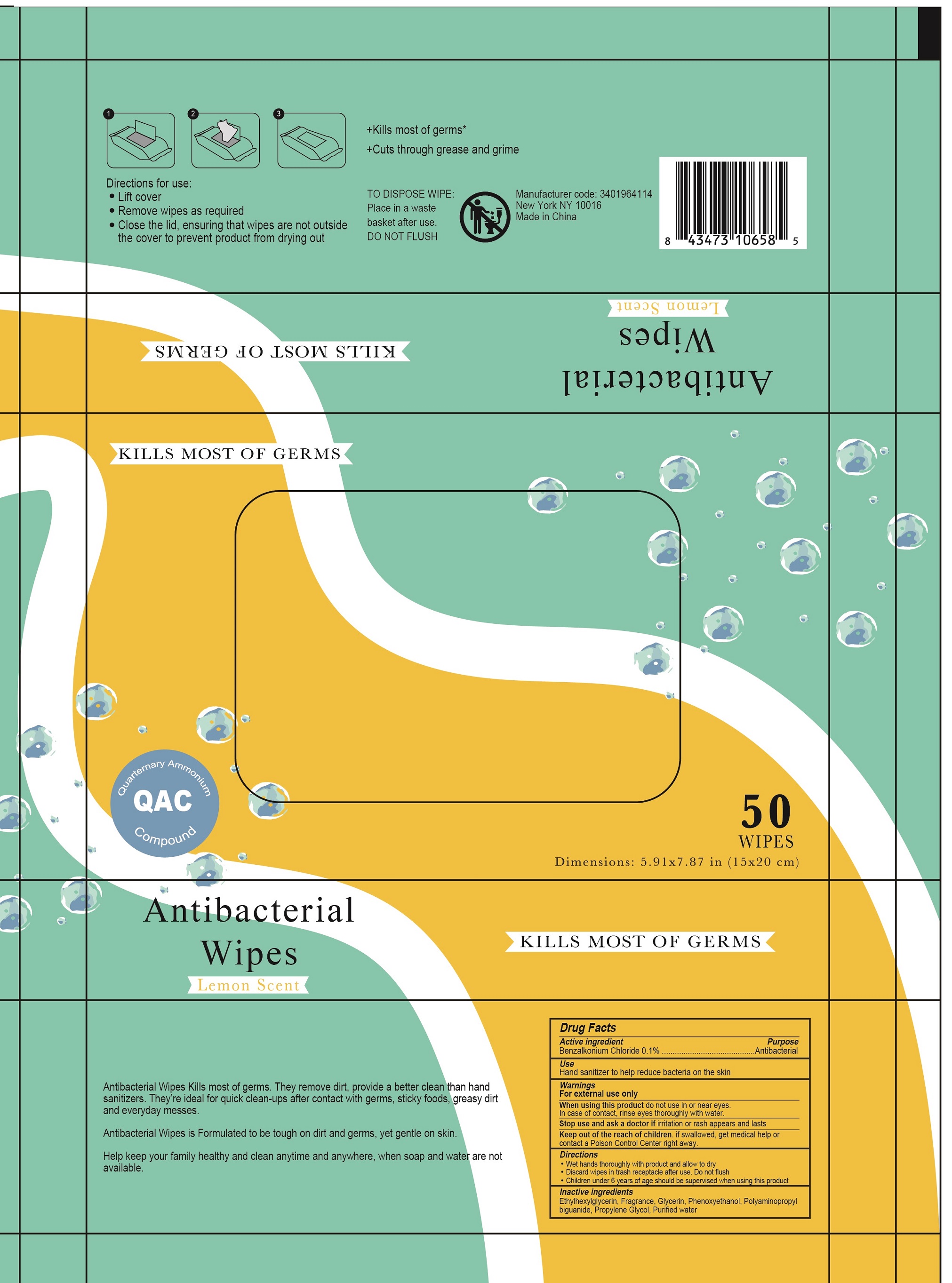
Mosaic Bath and Spa Antibacterial Wipes, Lemon Scent
Mosaic Bath and Spa Antibacterial Wipes, Lemon Scent by
Drug Labeling and Warnings
Mosaic Bath and Spa Antibacterial Wipes, Lemon Scent by is a Otc medication manufactured, distributed, or labeled by Anhui Yunzhiyu Daily Chemical Products Co., Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
MOSAIC BATH AND SPA ANTIBACTERIAL WIPES, LEMON SCENT- benzalkonium chloride cloth
Anhui Yunzhiyu Daily Chemical Products Co., Ltd
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Mosaic Bath and Spa Antibacterial Wipes, Lemon Scent
Warnings
For external use only
Directions
- Wet hands thoroughly with product and allow to dry
- Discard wipes in trash receptacle after use. Do not flush
- Children under 6 years of age should be supervised when using this product
| MOSAIC BATH AND SPA ANTIBACTERIAL WIPES, LEMON SCENT
benzalkonium chloride cloth |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Anhui Yunzhiyu Daily Chemical Products Co., Ltd (405732547) |
Revised: 4/2022
Document Id: ddceb04c-75cb-4c9c-e053-2a95a90a5770
Set id: ac22d700-7c6f-f127-e053-2995a90a7f25
Version: 3
Effective Time: 20220429
Anhui Y
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.