CARDALIS- spironolactone and benazepril hydrochloride tablet, chewable
CARDALIS by
Drug Labeling and Warnings
CARDALIS by is a Animal medication manufactured, distributed, or labeled by Ceva Sante Animale. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
Description:
CARDALIS (spironolactone and benazepril hydrochloride chewable tablets) for dogs contains two active ingredients, spironolactone and benazepril hydrochloride, in a fixed ratio of 8:1 respectively. CARDALIS is supplied as oblong half scored flavored chewable tablets in three sizes:
20 mg spironolactone and 2.5 mg benazepril hydrochloride, 40 mg spironolactone and 5 mg benazepril hydrochloride, and 80 mg spironolactone and 10 mg benazepril hydrochloride.
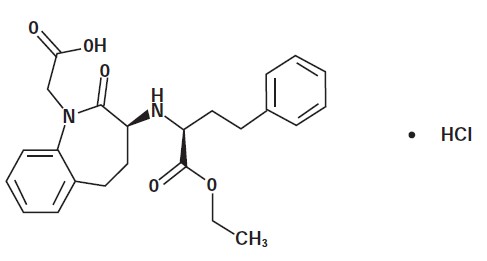
Benazepril belongs to the angiotensin-converting enzyme (ACE) inhibitor class of drugs. Benazepril hydrochloride empirical formula is C24H28N2O5 · HCl and the molecular weight is 460.95. The chemical name is 3-[[1-(ethoxy-carbonyl)-3-phenyl-(1S)-propyl]amino]-2,3,4,5-tetrahydro-2-oxo-1H-1-(3S)-benazepine-1-acetic acid monohydrochloride and the structural formula is shown below:
Spironolactone, and its active metabolites, act as specific aldosterone antagonists. Spironolactone empirical formula is C24H32O4S and the molecular weight is 416.57. The chemical name is 17-hydroxy-7α-mercapto-3-oxo-17α-pregn-4-ene-21-carboxylic acid y-lactone acetate and the structural formula is shown below:

- Indications:
-
Dosage and Administration:
CARDALIS administration should begin after pulmonary edema is stabilized. CARDALIS should be administered orally once daily at a dose of 0.9 mg/lb (2 mg/kg) spironolactone and 0.11 mg/lb (0.25 mg/kg) benazepril hydrochloride, according to dog body weight using a suitable combination of whole and/or half tablets. All tablet strengths are scored and the calculated dosage according to dog’s weight should be to the nearest half-tablet increment. CARDALIS should be administered with food.
-
Contraindications:
Do not administer CARDALIS in conjunction with non-steroidal anti-inflammatory drugs (NSAIDs) in dogs with renal insufficiency. Do not administer CARDALIS to dogs with hypoadrenocorticism (Addison’s Disease), hyperkalemia, or hyponatremia. Do not administer CARDALIS to animals with known hypersensitivity to ACE inhibitors or spironolactone.
-
Warnings:
Keep CARDALIS in a secure location out of reach of dogs, cats, and other animals to prevent accidental ingestion or overdose. In case of accidental overdose, induce vomiting, lavage the stomach (depending on risk assessment), and monitor electrolytes. Symptomatic therapy (e.g. fluid therapy) should be provided as medically necessary. CARDALIS is only for use in dogs with clinical evidence of heart failure.
Human Warnings: Not for use in humans. Keep this and all medications out of the reach of children. Consult a physician in case of ingestion by humans.
-
Precautions:
The safety and effectiveness of concurrent therapy of CARDALIS with pimobendan has not been evaluated.
Renal function and serum potassium levels should be evaluated prior to initiating treatment with CARDALIS. Regular monitoring of renal function and serum potassium levels is recommended as there may be an increased risk of hyperkalemia.
Dogs undergoing combined treatment with CARDALIS and NSAIDs should be adequately hydrated to avoid renal toxicity.
Concomitant use of desoxycorticosterone pivalate (DOCP) with spironolactone may counter the effect of DOCP as DOCP has an opposing mechanism of action to potassium-sparing diuretics like spironolactone. Closely monitor dogs receiving digoxin and spironolactone. Spironolactone decreases digoxin elimination and hence raises digoxin plasma concentration. This may result in digoxin toxicity.
Spironolactone and benazepril hydrochloride undergo extensive hepatic biotransformation. Care should be taken when using CARDALIS in dogs with hepatic dysfunction.
The safety of CARDALIS has not been evaluated in growing dogs. Spironolactone has an antiandrogenic effect and should be used with caution in growing dogs. The safety of CARDALIS has not been established in pregnant, lactating or breeding dogs.
-
Adverse Reactions:
A U.S. clinical field study comprised of a 360-day treatment period evaluated the safety and effectiveness of CARDALIS compared to benazepril hydrochloride in 569 client-owned dogs with left-sided AVVI. Table 1 summarizes the adverse reactions not directly related to the progression of disease that occurred in greater than 5% of the dogs treated with CARDALIS.
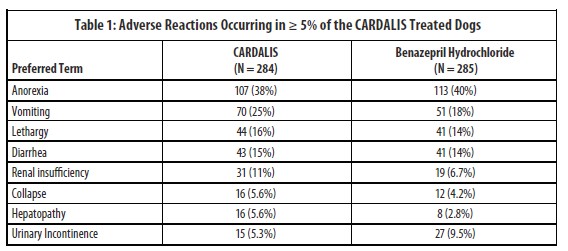
The following adverse events were seen in fewer than 5% of the study animals, in decreasing order: urine abnormalities, fluid in abdomen, ataxia, weight loss, digestive tract disorder, hypertension, electrolyte disorder, bronchitis, and hyperactivity.
Renal insufficiency was reported more frequently in dogs treated with CARDALIS. This finding may be also attributed to the concurrent administration of furosemide. The clinical pathology parameters associated with renal function were not statistically different between the treatment groups.
The incidence of death, including euthanasia and sudden death, was similar in dogs treated with CARDALIS or benazepril hydrochloride. In most cases, death was attributable to the progression of heart disease or the clinical signs associated with congestive heart failure. Deaths of unknown cause were presumed to be cardiac in nature.
Serum magnesium and potassium values were significantly higher in the CARDALIS group, although the mean values remained within the reference range and did not change significantly over time. These electrolyte changes are consistent with the potassium-sparing properties of spironolactone. One dog treated with CARDALIS was removed from the study at Day 7 because it developed hyperkalemia.
Contact Information: To report suspected adverse events or to request a copy of the Safety Data Sheet (SDS), please call Ceva Animal Health at 1-800-999-0297. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at www.fda.gov/reportanimalae.
-
Clinical Pharmacology:
Mechanism of Action: The main pharmacological target of spironolactone and benazepril is the renin-angiotensin-aldosterone system (RAAS) at different levels in the cascade. Spironolactone and its active metabolites (including 7-α-thiomethyl-spironolactone and Canrenone) act as specific antagonists of aldosterone (regardless of the source) by binding competitively to mineralocorticoid receptors located in the kidneys, heart and blood vessels.
Benazepril hydrochloride is a prodrug hydrolyzed in vivo into its active metabolite, benazeprilat. Benazeprilat is a highly potent and selective inhibitor of angiotensin-converting enzyme (ACE), thus preventing the conversion of inactive angiotensin I to active angiotensin II. This prevents deleterious effects of vasoconstriction and aldosterone release, including sodium and water retention, and vascular and myocardial remodeling. However, aldosterone release is not fully controlled by ACE inhibitors because angiotensin II is also produced by non-ACE pathways. Therefore, concomitant use of the aldosterone antagonist spironolactone and the ACE inhibitor benazepril hydrochloride inhibits both ACE and non-ACE pathways.
Pharmacokinetics: Spironolactone is rapidly and extensively metabolized in humans and experimental animals, with species differences in the metabolism and disposition. Spironolactone is metabolized by microsomal cytochrome P450 enzymes to a primary metabolite, canrenone, and a secondary metabolite, 7α-thiomethyl-spironolactone (TMS), which are used as markers for spironolactone in dog plasma. Systemic exposure to both metabolites was comparable when spironolactone was administered alone or in association with benazepril in dogs. Spironolactone absorption is affected by food and the dose should be administered consistently in fed condition. Spironolactone is mainly excreted as metabolites. After oral administration of radiolabeled spironolactone to the dog, 70% of the dose is recovered in feces and 20% in the urine.
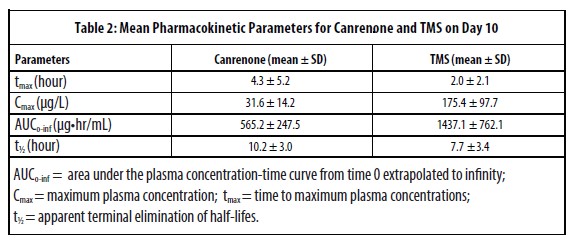
After multiple oral doses of 20 mg of spironolactone in association with 2.5 mg of benazepril in dogs (n=18, 9 male and 9 female) for 10 consecutive days (steady state), no accumulation was observed.
Benazepril hydrochloride is a prodrug which, after absorption from the gastrointestinal tract, is rapidly converted by nonspecific carboxyl esterases, mainly in the liver, into its active metabolite benazeprilat. Benazeprilat is itself poorly absorbed from the gastrointestinal tract and the overall bioavailability of benazeprilat after oral administration is estimated to be less than 7%.
After multiple oral doses of 2.5 mg of benazepril in association with 20 mg of spironolactone in dogs (n=18, 9 male and 9 female) for 10 consecutive days (steady state), no accumulation was observed. In dogs, benazeprilat is excreted approximately equally by both renal (45%) and hepatic (55%) routes.
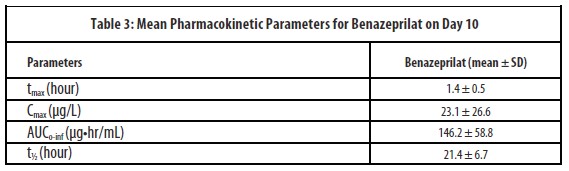
-
SUMMARY OF SAFETY AND EFFECTIVENESS
Effectiveness: The effectiveness of CARDALIS was evaluated in a well controlled U.S. multi-center, masked, randomized, 360-day field study in client-owned dogs. This study evaluated the effectiveness of CARDALIS in dogs diagnosed with congestive heart failure caused by left-sided AVVI compared to benazepril hydrochloride alone (active control).
Dogs ranged from 3 to 19 years of age and 5 to 155 lbs (2.3 to 70.5 kg) at enrollment. The most common breeds were mixed breed, Cavalier King Charles Spaniel, Chihuahua, Shih Tzu, Maltese, Dachshund, and Yorkshire Terrier. Enrolled dogs demonstrated radiographic evidence of congestive heart failure prior to enrollment or on Day 0 and exhibited clinical signs associated with left-sided AVVI, including exercise intolerance and/or dyspnea, echocardiographic evidence of left-atrial enlargement, moderate-to-severe mitral regurgitation, and presence of a left-sided cardiac murmur. Dogs with acquired heart disease other than left-sided AVVI, congenital heart defect, current positive heartworm antigen test, or syncope not related to heart disease, and dogs intended for breeding or known to be pregnant or lactating, were excluded.
A total of 569 dogs were treated with either CARDALIS (284 dogs) at a dose of 2 mg/kg spironolactone and 0.25 mg/kg benazepril hydrochloride once daily or benazepril hydrochloride alone (285 dogs) at a dose of 0.25 mg/kg once daily. Doses were administered with food or within 30 minutes of feeding. All dogs received concurrent administration of oral furosemide throughout the study (up to 8 mg/kg/day), to manage pulmonary edema. Digoxin and calcium channel blockers were allowed for control of supraventricular arrhythmias. The use of injectable furosemide was permitted during the study evaluation period only if used in place of an equivalent oral dose.
The rate of treatment failure was the primary effectiveness variable used to compare CARDALIS to benazepril hydrochloride alone. Treatment failure was defined as cardiac death or euthanasia (including death of unknown cause), recurrence or worsening of pulmonary edema, newly documented cardiogenic ascites, or clinical signs of congestive heart failure requiring administration of a furosemide dosage higher than 8 mg/kg/day. Failure rates at study Days 30, 90, 180, and 270 were also evaluated as secondary outcomes.
The rate of failure in the CARDALIS group estimated from the model analysis was statistically different (P = 0.0433) and numerically lower than that of the benazepril hydrochloride alone group, as summarized in Table 4.
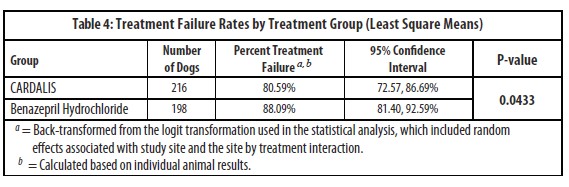
Further, the rate of failure in the CARDALIS group was significantly lower than the group administered benazepril hydrochloride alone at all evaluation periods past study Day 30. The dogs in the CARDALIS group exhibited a longer median time-to-failure when compared to the control group.
CARDALIS was safely administered in dogs concurrently receiving furosemide, digoxin, calcium channel blockers, antiparasitics, analgesics/anti-inflammatories, antibacterials, routine canine vaccines, respiratory treatments, and gastrointestinal treatments.
Palatability: During the field study, 233 dogs were offered CARDALIS once daily for 14 days. CARDALIS was accepted voluntarily, with or without food, in 87.6% of the 3178 reported doses.
Animal Safety: In a laboratory at safety study, 32 healthy one-year old Beagle dogs (16 males and 16 females) were randomly assigned to an untreated control group or were dosed orally with CARDALIS once daily for 26 weeks at 0, 1X, 3X, and 5X the maximum recommended daily dose (4 mg/kg spironolactone and 0.5 mg/kg benazepril hydrochloride). No dogs were removed and no unscheduled deaths occurred during the study. The dogs dosed with CARDALIS showed no test article-related effects on food or water consumption, body weights, electrocardiographic findings, or blood pressure. The dogs in the 5X group had lower mean heart rates compared to the other groups at the end of the study.
Increased mean serum potassium levels were found in dogs in all CARDALIS dose groups but remained within reference range. There was a dose-related decrease in red cell mass (mean red blood cell count, hemocrit and hemoglobin) but all variables remained within reference ranges. There were decreases in mean prostate weights in the 3X and 5X groups and signs of slight to marked atrophy of prostate glandular tissue. There was a thickening of the zona glomerulosa of adrenal glands in both male and female in the 3X and 5X treated animals.
Systemic exposure to the active metabolites of spironolactone (canrenone and 7-α-thiomethyl-spironolactone) and benazepril (benazeprilat) was shown at the three dose levels throughout the study, with no apparent gender effect. Canrenone and benazeprilat exposures were more than dose proportional at steady-state. There was no accumulation with repeated benazeprilat exposures; canrenone accumulation was 30%. Systemic exposure to 7-α-thiomethyl-spironolactone were variable by study day and dose of spironolactone; steady state, dose proportionality, and accumulation could not be determined.
- Storage Information:
-
How Supplied:
CARDALIS chewable tablets for dogs are available in 3 sizes of oblong half scored flavored tablets: 20 mg spironolactone and 2.5 mg benazepril hydrochloride, 40 mg spironolactone and 5 mg benazepril hydrochloride, and 80 mg spironolactone and 10mg benazepril hydrochloride. Each size is available in 30-count bottles enclosed in color coded packages.
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 20/2.5 mg Bottle Label
30 Tablets
Cardalis™
(spironolactone and benazepril hydrochloride chewable tablets)
20 mg/2.5 mg
Caution: Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
Read package insert before using this drug.
Manufactured for Ceva Animal Health, LLC, 1-800-999-0297
Store at 20-25C (68-77F).

-
PRINCIPAL DISPLAY PANEL - 20/2.5 mg Carton Label
Cardalis™
(spironolactone and benazepril hydrochloride chewable tablets)
20 mg/2.5 mg
Cardiac drug for oral use in dogs only
Caution: Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
Each chewable tablet contains 20 mg spironolactone and 2.5 mg benazepril hydrochloride
30 Tablets
Approved by FDA under NADA # 141-538

- PRINCIPAL DISPLAY PANEL - 40/5 mg Bottle Label
-
PRINCIPAL DISPLAY PANEL - 40/5 mg Carton Label
Cardalis™
(spironolactone and benazepril hydrochloride chewable tablets)
40 mg/5 mg
Cardiac drug for oral use in dogs only
Caution: Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
Each chewable tablet contains 20 mg spironolactone and 2.5 mg benazepril hydrochloride
30 Tablets
Approved by FDA under NADA # 141-538

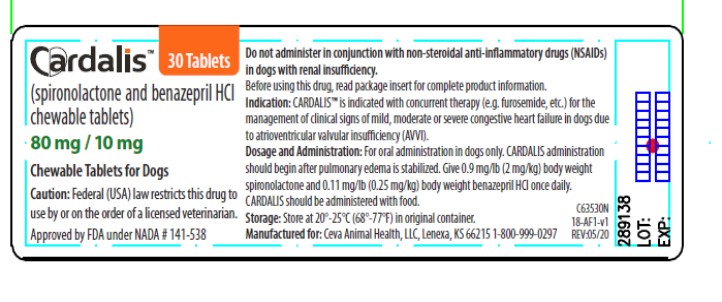
- PRINCIPAL DISPLAY PANEL - 80/10 mg Carton Label
-
PRINCIPAL DISPLAY PANEL - 80/10 mg Carton Label
Cardalis™
(spironolactone and benazepril hydrochloride chewable tablets)
80 mg/10 mg
Cardiac drug for oral use in dogs only
Caution: Federal (USA) law restricts this drug to use by or on the order of a licensed veterinarian.
Each chewable tablet contains 20 mg spironolactone and 2.5 mg benazepril hydrochloride
30 Tablets
Approved by FDA under NADA # 141-538

-
INGREDIENTS AND APPEARANCE
CARDALIS
spironolactone and benazepril hydrochloride tablet, chewableProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 13744-805 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SPIRONOLACTONE (UNII: 27O7W4T232) (SPIRONOLACTONE - UNII:27O7W4T232) SPIRONOLACTONE 20 mg BENAZEPRIL HYDROCHLORIDE (UNII: N1SN99T69T) (BENAZEPRILAT - UNII:JRM708L703) BENAZEPRIL HYDROCHLORIDE 2.5 mg Product Characteristics Color brown Score 2 pieces Shape BULLET Size 11mm Flavor MEAT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13744-805-30 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141538 01/01/2021 CARDALIS
spironolactone and benazepril hydrochloride tablet, chewableProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 13744-807 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SPIRONOLACTONE (UNII: 27O7W4T232) (SPIRONOLACTONE - UNII:27O7W4T232) SPIRONOLACTONE 40 mg BENAZEPRIL HYDROCHLORIDE (UNII: N1SN99T69T) (BENAZEPRILAT - UNII:JRM708L703) BENAZEPRIL HYDROCHLORIDE 5 mg Product Characteristics Color brown Score 2 pieces Shape BULLET Size 14mm Flavor MEAT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13744-807-30 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141538 01/01/2021 CARDALIS
spironolactone and benazepril hydrochloride tablet, chewableProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 13744-809 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SPIRONOLACTONE (UNII: 27O7W4T232) (SPIRONOLACTONE - UNII:27O7W4T232) SPIRONOLACTONE 80 mg BENAZEPRIL HYDROCHLORIDE (UNII: N1SN99T69T) (BENAZEPRILAT - UNII:JRM708L703) BENAZEPRIL HYDROCHLORIDE 10 mg Product Characteristics Color brown Score 2 pieces Shape BULLET Size 14mm Flavor MEAT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13744-809-30 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141538 01/01/2021 Labeler - Ceva Sante Animale (261126049) Registrant - Ceva Sante Animale (261126049)
Trademark Results [CARDALIS]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CARDALIS 90559965 not registered Live/Pending |
Ceva Santé Animale 2021-03-04 |
 CARDALIS 87711413 not registered Live/Pending |
Ceva Santé Animale S.A. 2017-12-07 |
 CARDALIS 79099065 4069321 Dead/Cancelled |
CEVA SANTE ANIMALE 2011-04-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.