LYMPHIR- denileukin diftitox-cxdl injection, powder, lyophilized, for solution
LYMPHIR by
Drug Labeling and Warnings
LYMPHIR by is a Prescription medication manufactured, distributed, or labeled by Citius Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use LYMPHIR™ safely and effectively. See full prescribing information for LYMPHIR.
LYMPHIR™ (denileukin diftitox-cxdl) for injection, for intravenous use
Initial U.S. Approval: 2024WARNING: CAPILLARY LEAK SYNDROME
See full prescribing information for complete boxed warning.
INDICATIONS AND USAGE
LYMPHIR is an IL2-receptor-directed cytotoxin indicated for the treatment of adult patients with relapsed or refractory Stage I-III cutaneous T-cell lymphoma (CTCL) after at least one prior systemic therapy. ( 1)
DOSAGE AND ADMINISTRATION
- Delay start of treatment cycle if serum albumin level is below 3 g/dL. ( 2.1)
- The recommended dosage of LYMPHIR is 9 mcg/kg/day actual body weight administered as an intravenous infusion on Days 1 through 5 of a 21-day cycle. ( 2.2)
- Administer premedication as recommended. ( 2.3)
- See full prescribing information for preparation and administration instructions. ( 2.5)
DOSAGE FORMS AND STRENGTHS
For injection: 300 mcg lyophilized cake in a single-dose vial. (3)
CONTRAINDICATIONS
None. ( 4)
WARNINGS AND PRECAUTIONS
- Visual Impairment: Monitor and evaluate for visual impairment throughout treatment. Withhold LYMPHIR until visual impairment resolves or permanently discontinue based on severity. ( 5.2)
- Infusion-Related Reactions: Monitor patients closely during infusions. Interrupt or discontinue for infusion-related reactions based on severity. ( 5.3)
- Hepatotoxicity: Monitor liver enzymes and bilirubin at baseline and during treatment as clinically indicated. ( 5.4)
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and to use effective contraception. ( 5.5, 8.1, 8.3)
ADVERSE REACTIONS
The most common adverse reactions (≥20%), including laboratory abnormalities, are increased transaminases, albumin decreased, nausea, edema, hemoglobin decreased, fatigue, musculoskeletal pain, rash, chills, constipation, pyrexia, and capillary leak syndrome. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Citius Oncology, Inc. at 1-844-459-6744 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
Lactation:Advise not to breastfeed. ( 8.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: CAPILLARY LEAK SYNDROME
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosing Instructions
2.2 Recommended Dosage
2.3 Recommended Premedications
2.4 Dosage Modifications for Adverse Reactions
2.5 Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS ANDPRECAUTIONS
5.1 Capillary Leak Syndrome
5.2 Visual Impairment
5.3 Infusion-Related Reactions
5.4 Hepatotoxicity
5.5 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2Pharmacodynamics
12.3 Pharmacokinetics
12.6Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: CAPILLARY LEAK SYNDROME
Capillary leak syndrome (CLS), including life-threatening or fatal reactions, can occur in patients receiving LYMPHIR. Monitor patients for signs and symptoms of CLS during treatment. Withhold LYMPHIR until CLS resolves, or permanently discontinue based on severity [see Dosage and Administration ( 2.1, 2.4) and Warnings and Precautions ( 5.1)] .
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosing Instructions
Prior to starting each treatment cycle, assess hepatic and renal function. If serum albumin is less than 3 g/dL, delay administration of LYMPHIR until serum albumin is greater than or equal to 3 g/dL [see Warnings and Precautions ( 5.1)] .
2.2 Recommended Dosage
The recommended dosage of LYMPHIR is 9 mcg/kg/day actual body weight administered as an intravenous infusion over 60 minutes on Days 1 through 5 of a 21-day treatment cycle. Administer LYMPHIR until disease progression or unacceptable toxicity.
2.3 Recommended Premedications
Administer premedications prior to starting a LYMPHIR infusion in Cycles 1 through 3, as outlined in Table 1, to reduce the risk of infusion-related reactions [see Warnings and Precautions ( 5.3)] .
Table 1. Premedication to be Administered to Patients Prior to LYMPHIR Infusion Treatment
CyclePremedication Dosage Administration Cycles 1-3
(optional thereafter)Antipyretic Oral acetaminophen 650 mg
or per local institutional
guidelinesAt least 30 minutes
prior to infusionAntihistamine Diphenhydramine 25 mg
intravenously or other
antihistamine per local
institutional guidelinesAt least 30 minutes
prior to infusionAntiemetic Per institutional guidelines At least 30 minutes
prior to infusionHydration 250 to 500 mL 0.9% Sodium
Chloride Injection
intravenously (or other
amount of fluid considered to
be most appropriate for thesubject’s condition)
At least 30 minutes
prior to infusionIf patients experience a Grade 2 or higher infusion reaction, premedicate at least 30 minutes prior to each subsequent infusion with a systemic steroid such as dexamethasone 4 mg (or equivalent) via slow intravenous push, for at least 3 cycles.
2.4 Dosage Modifications for Adverse Reactions
Dosage modifications for adverse reactions with LYMPHIR are shown in Table 2 below.
Table 2: Recommended Dosage Modifications for Adverse Reactions Adverse Reaction Severity* Dosage Modification Capillary leak syndrome (CLS) [see Warnings and Precautions ( 5.1)] Grade 2 - Withhold LYMPHIR until CLS resolves to Grade 1.
Grade 3 - Withhold LYMPHIR and provide supportive care until CLS resolves to Grade 1.
- Resume LYMPHIR at 50% dose.
- Consider steroid premedication for subsequent infusions.
- Permanently discontinue upon recurrence.
Grade 4 - Permanently discontinue LYMPHIR.
Visual impairment [see Warnings and Precautions ( 5.2)] Grade 1 or 2 - Consider withholding or discontinuing LYMPHIR as appropriate.
- Refer for ophthalmic evaluation.
Grade 3 or 4 - Withhold LYMPHIR until resolved to Grade 1 or baseline.
- Refer for ophthalmic evaluation.
- Permanently discontinue LYMPHIR if impairment does not resolve to Grade 1 or baseline.
Infusion-related reactions [see Warnings and Precautions ( 5.3)] Grade 2 or 3 Grade 4 or
Recurrent
Grade 3
- Permanently discontinue LYMPHIR.
Hepatotoxicity [see Warnings and Precautions ( 5.4)] Grade 2: ALT/AST 3 to
5 x upper limit
of normal
(ULN)- Maintain LYMPHIR dose
- Monitor at least weekly until return to
< 3 x ULN
Grade 3:
ALT/AST > 5
to 20 x ULN- Withhold LYMPHIR and monitor at least weekly until return to < 3 x ULN
- Resume LYMPHIR at same dose (first occurrence) or at a reduced dose (50%) for subsequent occurrence
Grade 4:
ALT/AST > 20
x ULN- Permanently discontinue LYMPHIR
Other Adverse Reactions [see Adverse Reactions ( 6.1)] Grade 3 - Withhold LYMPHIR until resolution of toxicity to Grade 1 or baseline.
Grade 4 - Permanently discontinue LYMPHIR as appropriate.
* Based on NCI CTCAE version 5.0
Restarting after Dosage Delay Due to Toxicity
Time Since the Last Dose Administered
Action for Next Dose
Less than or equal to 16 days
Restart a new cycle no earlier than 16
days after last dose of LYMPHIRMore than 16 days
Restart a new cycle of LYMPHIR
More than 42 days
Permanently discontinue LYMPHIR
2.5 Preparation and Administration
Reconstitute and further dilute LYMPHIR prior to administration. Administer as an intravenous infusion over 60 minutes. Use aseptic technique to prepare LYMPHIR.
LYMPHIR does not contain a preservative or anti-microbial agent. Care should be taken during preparation and administration of LYMPHIR to prevent inadvertent microbial contamination.
Reconstitution
- Calculate the dose (mcg), total volume (mL) of solution required, and number of vials of LYMPHIR needed based on the patient’s actual body weight (kg). More than one vial may be needed for a full dose.
- Remove the vial(s) of LYMPHIR from the refrigerator and allow to stand for approximately 30 minutes to reach to room temperature [20°C to 25°C (68°F to 77°F)].
- Reconstitute each LYMPHIR vial with 2.1 mL of Sterile Water for Injection to obtain a final concentration of 150 mcg/mL. If more than one vial is needed to make a complete dose, use a separate syringe and needle for each vial.
- Gently swirl the vial. Do not shake.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. The reconstituted solution should be clear and colorless. Discard if the solution is not clear, colorless, and contains particulate matter.
- Use the reconstituted LYMPHIR solution immediately to prepare the drug product.
Dilution
- Use only plastic syringes or soft plastic intravenous bags. Do notuse glass containers.
- Maintain concentration of LYMPHIR at 15 mcg/mL or higher during all steps in the preparation of the solution for intravenous infusion.
- Withdraw the calculated dose from the vial(s) with a sterile syringe and inject it into an empty intravenous bag.
- Dilute with 0.9% Sodium Chloride Injection to final concentration of 15 mcg/mL or higher.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if the prepared solution is cloudy or particulates are present.
- If not used immediately, store the diluted LYMPHIR solution at room temperature [20°C to 25°C (68°F to 77°F)] for up to 4 hours. Protect diluted solution from light.
Administration
- Administer infusion solution intravenously over 60 minutes through an intravenous line using a syringe pump or intravenous infusion bag. Do not administer through in-line filter.
- Do not mix LYMPHIR with other drugs or administer other drugs through the same infusion line.
- At the end of the infusion, discard any empty or unused portions of LYMPHIR immediately.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS ANDPRECAUTIONS
5.1 Capillary Leak Syndrome
LYMPHIR can cause capillary leak syndrome (CLS), including life-threatening or fatal reactions. CLS was defined in the clinical trials as the occurrence of at least 2 of the following symptoms at any time during LYMPHIR therapy: hypotension, edema, and serum albumin <3 g/dL. These symptoms were not required to occur simultaneously to be characterized as capillary leak syndrome.
As defined, CLS occurred in 27% of patients in the pooled population across 3 clinical trials, including 8% with Grade 3. There was one (0.8%) fatal occurrence of CLS. Of the patients with CLS, 22% had recurrence. The majority of CLS events (81%) occurred within the first 2 cycles of treatment. The median time to onset from Cycle 1, Day 1 was 6.5 days (range: 1 to 77), the median duration of CLS was 14 days (range: 2 to 40), and 75% of patients had resolution. The most common symptoms included edema, hypoalbuminemia, and hypotension. Pleural effusion, pericardial effusion, and dehydration also occurred.
Regularly assess patients for weight gain, new onset or worsening of edema, dyspnea, and hypotension (including orthostatic changes). Monitor serum albumin levels prior to the initiation of each cycle of therapy and more often as clinically indicated.
Withhold, reduce dose, or permanently discontinue based on severity. If LYMPHIR is withheld, resume LYMPHIR following resolution of CLS and when serum albumin is greater than or equal to 3 g/dL [see Dosage and Administration ( 2.1and 2.4)] .
5.2 Visual Impairment
LYMPHIR can cause serious visual impairment, including changes in visual acuity and color vision. In the pooled population across 3 clinical trials, visual impairment occurred in 9%, with Grade 1 in 8% and Grade 2 in 1%. The most commonly reported symptom was blurred vision. Of the patients with visual impairment, 67% had resolution of their visual impairment.
Perform baseline ophthalmic examination and monitor as clinically indicated. If patients experience symptoms of visual impairment, such as changes in visual acuity, changes in color vision, or blurred vision, refer for ophthalmologic evaluation.
Withhold LYMPHIR until visual impairment resolves or permanently discontinue based on severity [see Dosage and Administration ( 2.4)] .
5.3 Infusion-Related Reactions
LYMPHIR can cause serious infusion-related reactions. Infusion-related reactions were reported in 69% of patients in the pooled population across 3 clinical trials of patients who received LYMPHIR, with Grade 3 infusion-related reactions in 3.4% [see Adverse Reactions ( 6.1)] . Eighty-three percent of infusion-related reactions occurred in Cycles 1 and 2. The most common symptoms included nausea, fatigue, chills, musculoskeletal pain, vomiting, fever, and arthralgia.
Premedicate patients for the first three cycles prior to starting a LYMPHIR infusion [see Dosage and Administration ( 2.3)] . Monitor patients frequently during infusion. For Grade 2 or higher infusion reactions, premedicate at least 30 minutes prior to each subsequent infusion with a systemic steroid for at least 3 cycles [see Dosage and Administration ( 2.3)] .
Interrupt or discontinue LYMPHIR based on severity [see Dosage and Administration ( 2.4)]. Institute appropriate medical management.
5.4 Hepatotoxicity
LYMPHIR can cause hepatotoxicity. In the pooled safety population, elevated ALT occurred in 70% of patients, with Grade 3 ALT occurring in 22%; elevated AST occurred in 64% of patients, with Grade 3 AST elevation occurring in 9%. For Grade 3 events, median time to onset was 8 days (range: 1 to 15 days); median time to resolution was 15 days (range: 7 to 50 days); all cases of Grade 3 ALT or AST elevations resolved [see Adverse Reactions ( 6.1)]. Elevated total bilirubin occurred in 5% of patients, with Grade 3 occurring in 0.9%.
Monitor liver enzymes and bilirubin at baseline and during treatment as clinically indicated. Withhold, reduce dose, or permanently discontinue LYMPHIR based on severity [see Dosage and Administration ( 2.4)] .
5.5 Embryo-Fetal Toxicity
Based on its mechanism of action, LYMPHIR can cause fetal harm when administered to a pregnant woman. Verify the pregnancy status of females of reproductive potential prior to the initiation of LYMPHIR. Advise pregnant women of the potential risk to the fetus. Advise females of reproductive potential to use effective contraception during treatment and for 7 days following the last dose of LYMPHIR [see Use in Specific Populations ( 8.1, 8.3)] .
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the label:
- Capillary Leak Syndrome [see Warnings and Precautions ( 5.1)]
- Visual Impairment [see Warnings and Precautions ( 5.2)]
- Infusion-Related Reactions [see Warnings and Precautions ( 5.3)]
- Hepatotoxicity [see Warnings and Precautions ( 5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The pooled safety population described in the WARNINGS AND PRECAUTIONS reflect exposure to LYMPHIR as a single agent in 119 patients with CTCL across 3 clinical trials. Patients received treatment with LYMPHIR as an intravenous infusion at 9 mcg/kg daily from Day 1 through Day 5 of each 21-day cycle until disease progression or unacceptable toxicity. Among 119 patients who received LYMPHIR the median number of cycles received was 5 (range: 1 to 42), with 13% exposed for 6 months or longer.
In this pooled safety population, the most common (≥ 20%) adverse reactions, including laboratory abnormalities, were increased transaminases (70%), albumin decreased (53%), nausea (40%), edema (35%), hemoglobin decreased (34%), fatigue (30%), musculoskeletal pain (26%), rash (23%), chills (22%), constipation (22%), pyrexia (21%), and capillary leak syndrome (20%).
Relapsed or Refractory Stage I-III CTCL
Study 302
The safety of LYMPHIR was evaluated in Study 302, an open-label, single-arm, multicenter trial that included 69 patients with relapsed or refractory Stage I-III CTCL [see Clinical Studies ( 14)] . Patients received treatment with LYMPHIR 9 mcg/kg daily from Day 1 through Day 5 of each 21-day cycle. Treatment was administered until disease progression or unacceptable toxicity. The median number of LYMPHIR cycles was 6 (range: 1 to 42).
The median age of patients was 64 years (range: 28 to 87 years), 49% were 65 years of age or older, 65% were men, 72% were White, 19% were Black or African American, 1.4% were Asian, and 14% were Hispanic or Latino.
Serious adverse reactions occurred in 38% of patients who received LYMPHIR. Serious adverse reactions in > 2% of patients included capillary leak syndrome (10%), infusion-related reaction (9%), sepsis (7%), skin infection (2.9%), pyrexia (2.9%), and rash (2.9%).
Permanent discontinuation of LYMPHIR due to an adverse reaction occurred in 12% of patients. Adverse reactions resulting in permanent discontinuation of LYMPHIR included capillary leak syndrome, infusion-related reaction, renal insufficiency, respiratory failure, and sepsis.
Dosage interruptions of LYMPHIR due to an adverse reaction occurred in 38% of patients. Adverse reactions requiring dosage interruption of LYMPHIR included capillary leak syndrome, infusion-related reaction, weight increase, nausea, and tachycardia.
Table 3 summarizes the adverse reactions in Study 302.
Table 3: Adverse Reactions (≥ 10%) in Patients with Relapsed or Refractory Stage I-III CTCL Who Received LYMPHIR in Study 302 Adverse Reaction LYMPHIR
N=69All Grades
(%)Grade 3 or 4
(%)Gastrointestinal disorders Nausea 43 1.4 # Diarrhea 19 0 Vomiting 13 0 Constipation 12 0 General disorders and administration site conditions Fatigue a 38 0 Edema b 33 1.4 Chills 27 1.4 Pyrexia 16 1.4 Musculoskeletal and connective tissue disorders Musculoskeletal pain c 27 2.9 Arthralgia 12 0 Nervous system disorders Headache d 25 0 Dizziness 13 0 Mental status changes e 13 0 Injury, poisoning and procedural complications Infusion-related reaction 25 6 Skin and subcutaneous tissue disorders Rash f 23 6 Pruritis 19 6 Vascular disorders Capillary leak syndrome 17 6 Metabolism and nutrition disorders Decreased appetite 13 1.4 Eye disorders Blurred vision 13 0 Investigations Weight increased 13 0
Infections and infestations Skin infection g 13 1.4 Renal and urinary disorders Renal insufficiency h 12 2.9 Psychiatric disorders Insomnia 10 0 #Only Grade 3 adverse reaction occurred
aIncludes fatigue, asthenia, and lethargy
bIncludes edema, edema peripheral, generalized edema, face edema, swelling face, peripheral swelling
cIncludes musculoskeletal pain, back pain, neck pain, pain in extremity, myalgia, bone pain
dIncludes headache, migraine
eIncludes amnesia, confusional state, delirium, memory impairment, disturbance in attention, somnolence, cognitive disorder
fIncludes rash, drug eruption, erythema, rash maculo-papular, rash papular, rash pustular, rash pruritic, dermatitis exfoliative generalized, acute generalized exanthematous pustulosis
gIncludes skin infection, skin bacterial infection, staphylococcal skin infection, impetigo
hIncludes acute kidney injury, blood creatinine increasedClinically relevant adverse reactions in < 10% of patients who received LYMPHIR included sepsis and peripheral neuropathy.
Table 4 summarizes select laboratory abnormalities in Study 302.
Table 4: Select Laboratory Abnormalities (≥ 10%) that Worsened from Baseline in Patients with Relapsed or Refractory Stage I-III CTCL who Received LYMPHIR in Study 302 Laboratory Abnormality a,b Any Grade c
(%) bGrade 3 or 4 c
(%)Chemistry Alanine aminotransferase increased 66 13 Aspartate aminotransferase increased 60 4.5 Albumin decreased 43 1.5 Creatine phosphokinase increased d 22 3 Alkaline phosphatase increased 18 0 Hematology Lymphocyte count decreased 52 20 Hemoglobin decreased 34 1.5 aGraded according to NCI CTCAE version 5.0
bThe denominator used to calculate the rate was 67 based on the number of patients with a baseline value and at least one post-treatment value
cPercentage of patients with an increase of at least 1 CTCAE grade from baseline to the worst post-baseline value of any grade, or to the worst post-baseline value that is Grade 3 or 4
dThe denominator used to calculate the rate was based on 36 patients with a baseline value and at least one post-treatment value.6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of denileukin diftitox. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Thyroid conditions: hyperthyroidism, thyroiditis, thyrotoxicosis, and hypothyroidism.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on its mechanism of action, LYMPHIR can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology ( 12.1)]. There are no available data on the use of LYMPHIR in pregnant women to evaluate for a drug-associated risk. No animal reproductive and developmental toxicity studies have been conducted with denileukin diftitox.
Denileukin diftitox-cxdl causes depletion of regulatory T lymphocytes (Treg), immune activation, and capillary leak syndrome, compromising pregnancy maintenance. Advise pregnant women of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies are 2-4% and 15-20%, respectively.
8.2 Lactation
Risk Summary
No data are available regarding the presence of denileukin diftitox-cxdl in human milk, the effects on the breastfed child, or on milk production. Because of the potential for serious adverse reactions in breastfed children, advise women not to breastfeed during treatment with LYMPHIR and for 7 days after the last dose.
8.3 Females and Males of Reproductive Potential
Based on its mechanism of action, LYMPHIR can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations ( 8.1)] .
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to initiating LYMPHIR [see Use in Specific Populations ( 8.1)] .
Contraception
Females
Advise females of reproductive potential to use effective contraception during treatment with LYMPHIR and for 7 days after the last dose.
Infertility
Males
Based on findings in rats, male fertility may be compromised by treatment with LYMPHIR [see Nonclinical Toxicology ( 13.1)] . The reversibility of the effect on fertility is unknown.
8.4 Pediatric Use
Safety and effectiveness of LYMPHIR in pediatric patients have not been established.
8.5 Geriatric Use
Of the 69 patients with Stage I-III relapsed or refractory CTCL who received LYMPHIR, 34 patients (49%) were 65 years of age and older and 10 patients (14%) were 75 years of age and older [see Clinical Studies ( 14)] . Clinical studies of LYMPHIR did not include sufficient numbers of patients 65 years of age and older to determine whether they respond differently from younger adult patients.
-
11 DESCRIPTION
Denileukin diftitox-cxdl, an IL2-receptor-directed cytotoxin, is a recombinant DNA-derived fusion protein composed of the amino acid sequences for diphtheria toxin fragments A and B (Met 1-Thr 387)-His and the sequence for human interleukin-2 (IL-2; Ala 1-Thr 133). It is produced in an E. coliexpression system and has a molecular weight of 58 kD. Neomycin is used in the fermentation process but is undetectable in the final product.
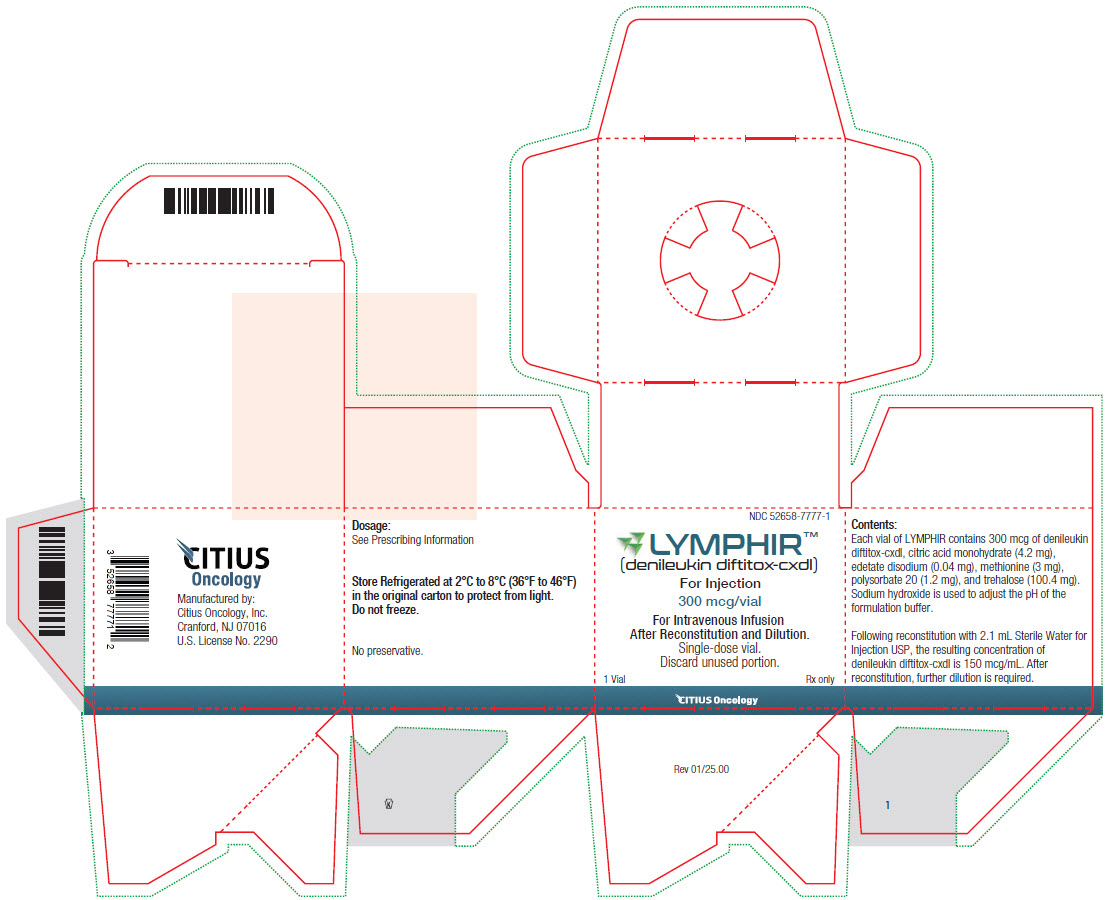
LYMPHIR (denileukin diftitox-cxdl) for injection is supplied as a sterile, white, lyophilized cake in a single-dose vial for intravenous use after reconstitution and dilution. Each vial of LYMPHIR contains 300 mcg of denileukin diftitox-cxdl, citric acid monohydrate (4.2 mg), edetate disodium (0.04 mg), methionine (3 mg), polysorbate 20 (1.2 mg), and trehalose (100.4 mg). Sodium hydroxide is used to adjust the pH of the formulation buffer. Following reconstitution with 2.1 mL of Sterile Water for Injection, USP, the resulting concentration of denileukin diftitox-cxdl is 150 mcg/mL at pH approximately 7.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Denileukin diftitox-cxdl is a fusion protein designed to direct the cytocidal action of diphtheria toxin (DT) to cells which express the IL-2 receptor. After uptake into the cell, the DT fragment is cleaved and the free DT fragments inhibit protein synthesis, resulting in cell death. Denileukin diftitox-cxdl demonstrated the ability to deplete immunosuppressive regulatory T lymphocytes (Tregs) and antitumor activity through a direct cytocidal action on IL-2R-expressing tumors.
12.2Pharmacodynamics
Higher denileukin diftitox-cxdl exposures were associated with higher incidence of capillary leak syndrome, hepatotoxicity, treatment discontinuations due to adverse reactions, and adverse reactions Grade ≥3 following administration of LYMPHIR. Denileukin diftitox-cxdl time course of pharmacodynamic response is unknown.
12.3 Pharmacokinetics
Following a single dose of denileukin diftitox-cxdl 9 mcg/kg via 1-hr infusion on Cycle 1, Day 1 in patients with CTCL, the geometric mean (coefficient of variation [CV]%) maximum serum concentration (C max) was 94.4 ng/mL (77%) and area under the concentration over time curve (AUC 0-inf) was 20700 ng· min/L (60%). There is no accumulation after repeated daily dosing.
Distribution
The geometric mean (CV%) volume of distribution of denileukin diftitox-cxdl is 5.0 L (43%) on Cycle 1, Day 1.
Elimination
The geometric mean (CV%) clearance is 36.5 mL/min (73%) after the first dose of denileukin diftitox-cxdl at the recommended dose level. The arithmetic mean (CV%) denileukin diftitox-cxdl terminal half-life is 112 minutes (31%) on Cycle 1, Day 1.
Metabolism
Denileukin diftitox-cxdl is expected to be metabolized into small peptides by catabolic pathways.
Specific Populations
No clinically significant differences in the pharmacokinetics of LYMPHIR were observed based on age (23 to 86 years), body weight (39 kg to 148 kg), sex, race (White, African American/Black, Asian), ECOG status (0 to 2), mild to moderate renal impairment (estimated creatinine clearance [CLcr] by Cockcroft–Gault equation: 30 to 89 mL/min), or mild hepatic impairment (total bilirubin less than or equal to upper limit of normal [ULN] and AST greater than ULN, or total bilirubin 1 to 1.5 times ULN and any AST). The effect of moderate or severe hepatic impairment (total bilirubin greater than 1.5 times ULN with any AST) and severe renal impairment (CLcr 15 to 29 mL/min) on denileukin diftitox-cxdl pharmacokinetics is unknown.
12.6Immunogenicity
The observed incidence of anti-drug antibodies (ADAs) is highly dependent on the sensitivity and specificity of the methods. Differences in ADA assay methods may preclude meaningful comparisons of the incidence of ADAs in the studies of denileukin diftitox-cxdl.
In Study 302, among patients with any stage CTCL who received LYMPHIR 9 mcg/kg/day (median duration of treatment: 6 cycles),
- Of the 90 subjects with immunogenicity results at baseline, 74% (67/90) of subjects had pre-existing antibodies to denileukin diftitox-cxdl and 7.8% (7/90) of the subjects had neutralizing anti-denileukin diftitox-cxdl antibodies. The high prevalence of ADA at baseline was anticipated due to vaccination against diphtheria toxin. Among these subjects, 5.6% (5/90) of the subjects had ADAs against IL-2 but none had neutralizing antibodies against IL-2.
- Of the 84 subjects evaluable for treatment-emergent (TE) ADA across all cycles after treatment with LYMPHIR, 88% (74/84) and 82% (69/84) of subjects developed treatment-emergent ADAs against denileukin diftitox-cxdl and IL-2, respectively. Among these subjects, 80% (67/84) developed neutralizing antibodies against denileukin diftitox-cxdl and 13% (11/84) developed neutralizing antibodies against the IL-2 domain of denileukin diftitox-cxdl.
- Treatment with LYMPHIR leads to an increase in anti-denileukin diftitox-cxdl and anti-IL-2 antibody titers. In subjects with treatment-emergent ADA during Study 302, the median anti-denileukin diftitox-cxdl antibody titers were 182, 260, 153, and 94 times higher than baseline at C2D1, C3D1, C5D1, and C8D1 and the anti-IL2 antibody titers were 2, 49, 437, and 1312 times higher than baseline at these same visits.
Anti-Drug Antibody Effects on Pharmacokinetics
The presence of ADA is associated with a reduction in the measured serum concentration. In a subset of patients with treatment-emergent anti-denileukin diftitox-cxdl and/or anti-IL-2 antibodies during Study 302, the geometric mean ratio for denileukin diftitox-cxdl C maxand AUC (0-t)is 37% and 19% in Cycle 3 compared to Cycle 1, and is 30% and 15% in Cycle 5 compared to Cycle 1. The decrease in denileukin diftitox-cxdl concentration does not appear to adversely impact safety and efficacy.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
There have been no studies to assess the carcinogenic potential of denileukin diftitox-cxdl. Denileukin diftitox-cxdl showed no evidence of mutagenicity in the Ames test and the chromosomal aberration assay. There have been no studies to assess the effect of denileukin diftitox on fertility. In a 14-week repeat-dose toxicology study in rats, denileukin diftitox-cxdl caused toxicity to male reproductive organs including atrophy of the epididymis, prostate gland, and testes and single cell necrosis in the epididymis.
-
14 CLINICAL STUDIES
Relapsed or Refractory CTCL
The efficacy of LYMPHIR was evaluated in Study 302 (NCT01871727), an open-label, single-arm, multicenter trial in patients with relapsed or refractory Stage I to IV CTCL. Eligible patients were required to have expression of CD25 on ≥ 20% of biopsied malignant cells by immunohistochemistry .The study excluded patients with significant cardiac disease or uncontrolled infections. Patients received LYMPHIR at 9 mcg/kg as an intravenous infusion daily from Day 1 through Day 5 of each 21-day cycle. Patients continued to receive LYMPHIR until disease progression or unacceptable toxicity.
The efficacy population includes 69 patients with relapsed or refractory Stage I to III CTCL. Of the 69 patients, the median age was 64 years (range: 28 to 87 years), 65% were male, 73% were White, 19% Black or African American, 1% Asian, and 14% Hispanic or Latino. The CTCL disease stage was IA in 7%, IB in 23%, IIA in 13%, IIB in 35%, IIIA in 12%, and IIIB in 10%. The median number of prior therapies was 4 (range: 1 to 18), including both skin-directed and systemic therapies. Prior therapies included photodynamic therapy (56%), total skin electron beam therapy (42%), systemic retinoids (49%), methotrexate/pralatrexate (49%), histone deacetylase inhibitor (35%), brentuximab vedotin (26%) and mogamulizumab (12%).
Efficacy was established based on objective response rate (ORR), according to ISCL/EORTC Global Response Score (GRS) per Independent Review Committee (Olsen 2011). Efficacy results are shown in Table 5.
Table 5: Efficacy Results of Study 302 Efficacy Endpoint LYMPHIR 9 mcg/kg/day (N=69)
ORR (GRS)% a
(95% CI b)36%
(25, 49)Complete Response 9% Partial Response 27% Duration of Response c Range, months
Duration ≥ 6 months, n (%)
Duration ≥ 12 months, n (%)3.0+, 23.5+
13 (52%)
5 (20%)aORR, objective response rate per Olsen, et al (2011) Global Response Score (GRS), by Independent Review Committee (IRC).
bCI, confidence interval
cThe median (95% CI) DOR using Kaplan-Meier (KM) estimate was not estimable (NE) among the 25 subjects due to censoring.Median time to response was 1.4 months (range: 0.7 to 5.6 months).
Among responders, the median follow-up for duration of response was 6.5 months (range: 3.5+, 23.5+ months).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
LYMPHIR (denileukin diftitox-cxdl) for injection is supplied as a sterile, white, lyophilized cake for reconstitution in a single-dose vial containing 300 mcg denileukin diftitox-cxdl. Each carton contains one vial:
NDC: 52658-7777-1
Storage and Handling
Store LYMPHIR in a refrigerator between 2°C to 8°C (36°F to 46°F) in the original carton to protect from light. Do not freeze.
-
17PATIENT COUNSELING INFORMATION
Discuss the following with patients prior to and during treatment with LYMPHIR.
Capillary Leak Syndrome
Inform patients of the signs and symptoms of capillary leak syndrome. Advise patients to contact their healthcare provider immediately if any signs or symptoms of capillary leak syndrome occur, including edema or change in blood pressure. Instruct patients to weigh themselves daily and report weight gain [see Warnings and Precautions ( 5.1)] .
Visual Impairment
Inform patients that if they experience symptoms of visual impairment, such as changes in visual acuity, changes in color vision, or blurred vision, they should report it promptly [see Warnings and Precautions ( 5.2)] .
Infusion-Related Reactions
Inform patients of the signs and symptoms of infusion-related reactions. Advise patients to contact their healthcare provider immediately if any signs or symptoms of infusion-related reaction occur, including fever, chills, breathing problems, chest pain, or hives [see Warnings and Precautions ( 5.3)] .
Hepatotoxicity
Advise patients that liver enzyme elevations can occur and that they should report symptoms that may indicate liver toxicity, including fatigue, anorexia, right upper abdominal discomfort, dark urine, or jaundice [see Warnings and Precautions ( 5.4)].
Embryo-Fetal Toxicity
Advise pregnant women and female patients of reproductive potential of the potential risk to a fetus. Advise female patients of reproductive potential to inform their healthcare provider of a known or suspected pregnancy and to use effective contraception for 7 days after the last dose [see Warnings and Precautions ( 5.5), Use in Specific Populations ( 8.1, 8.3)] .
Lactation
Advise women not to breastfeed during treatment with LYMPHIR and for 7 days after the last dose [see Use in Specific Populations ( 8.2)] .
Manufactured by:
Citius Oncology, Inc.
Cranford, NJ 07016
US License No. 2290LYMPHIR™ is the trademark of Citius Oncology, Inc.
© 2024 Citius Oncology, Inc.
-
PRINCIPAL DISPLAY PANEL
NDC: 52658-7777-1
LYMPHIR™
(denileukin diftitox-cxdl)
For Injection
300 mcg/vialFor Intravenous Infusion
After Reconstitution and Dilution.
Single-dose Vial.
Discard unused portion.1 Vial Rx Only
-
INGREDIENTS AND APPEARANCE
LYMPHIR
denileukin diftitox-cxdl injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 52658-7777 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DENILEUKIN DIFTITOX (UNII: 25E79B5CTM) (DENILEUKIN DIFTITOX - UNII:25E79B5CTM) DENILEUKIN DIFTITOX 300 ug in 2 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 4.2 mg in 2 mL EDETATE DISODIUM (UNII: 7FLD91C86K) 0.04 mg in 2 mL METHIONINE (UNII: AE28F7PNPL) 3 mg in 2 mL POLYSORBATE 20 (UNII: 7T1F30V5YH) 1.2 mg in 2 mL TREHALOSE DIHYDRATE (UNII: 7YIN7J07X4) 100.4 mg in 2 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52658-7777-1 1 in 1 CARTON 03/01/2025 1 2 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761312 03/01/2025 Labeler - Citius Pharmaceuticals, Inc. (872692764)
Trademark Results [LYMPHIR]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 LYMPHIR 97871965 not registered Live/Pending |
Citius Pharmaceuticals, Inc. 2023-04-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.