These highlights do not include all the information needed to use AEROSPAN® safely and effectively. See full prescribing information for AEROSPAN®. AEROSPAN® (flunisolide) inhalation aerosol, for oral inhalation use Initial U.S. Approval: 1981
Aerospan by
Drug Labeling and Warnings
Aerospan by is a Prescription medication manufactured, distributed, or labeled by Meda Pharmaceuticals. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
AEROSPAN- flunisolide aerosol, metered
Meda Pharmaceuticals
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use AEROSPAN® safely and effectively. See full prescribing information for AEROSPAN®.
AEROSPAN® (flunisolide) inhalation aerosol, for oral inhalation use Initial U.S. Approval: 1981 INDICATIONS AND USAGEDOSAGE AND ADMINISTRATIONFor oral inhalation only. Inhaler includes a built-in spacer. Do not use with external spacers or holding chambers. Shake well prior to each inhalation. (2.1)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSThe most common adverse reactions are pharyngitis, rhinitis, headache, sinusitis, and increased cough. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Meda Pharmaceuticals Inc. at 855-653-6325 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 4/2018 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
AEROSPAN inhalation aerosol is indicated for the maintenance treatment of asthma as prophylactic therapy in adult and pediatric patients 6 years of age and older.
Important Limitations of Use
- AEROSPAN is NOT indicated for the relief of acute bronchospasm.
- AEROSPAN is NOT indicated in children less than 6 years of age.
2 DOSAGE AND ADMINISTRATION
2.1 Administration Information
AEROSPAN should be administered by the orally inhaled route in patients aged 6 years and older. Shake well prior to each inhalation. After inhalation, the patient should rinse his/her mouth with water without swallowing to help reduce the risk of oropharyngeal candidiasis.
Patients should be instructed on the proper use of their inhaler. AEROSPAN contains a built-in spacer. Do not use with any external spacer or holding chamber devices. Instruct patients to prepare the inhaler for use by pulling the built-in purple actuator out from the gray spacer and snapping into an "L" shape prior to use. Pediatric patients should administer AEROSPAN under adult supervision.
2.2 Recommended Dosage
Adults and Adolescents 12 Years of Age and Older
The starting dosage is based on previous asthma therapy and disease severity, including consideration of the patients’ current control of asthma symptoms and risk of future exacerbation. The recommended starting dosage for patients aged 12 years and older who are not on an inhaled corticosteroid is 160 mcg twice daily, approximately 12 hours apart. For other patients, and for patients who do not respond adequately to Aerospan 160 mcg after 2 weeks of therapy, increasing the dosage may provide additional asthma control. The maximum recommended dosage is 320 mcg twice daily.
Pediatric Patients 6 to 11 Years
The starting dosage is based on previous asthma therapy and disease severity, including consideration of the patients’ current control of asthma symptoms and risk of future exacerbation. The recommended starting dosage for patients aged 6 to 11 years who are not on an inhaled corticosteroid is 80 mcg twice daily, approximately 12 hours apart. For other patients, and for patients who do not respond adequately to Aerospan 80 mcg after 2 weeks of therapy, increasing the dosage may provide additional asthma control. The maximum recommended dosage for patients 6 to 11 years of age is 160 mcg twice daily.
General Dosing Recommendations
If symptoms arise between doses, an inhaled short-acting beta2-agonist should be used for immediate relief.
The onset and degree of symptom relief with orally inhaled corticosteroids is usually apparent within 2-4 weeks after the start of treatment, and varies with individual patients. The time to improvement in asthma control was not evaluated in clinical studies with AEROSPAN.
If a dosage regimen of AEROSPAN and additional therapeutic options, e.g., increasing the AEROSPAN dosage, initiating an inhaled corticosteroid and long-acting beta2-agonist (LABA) combination product, or initiating oral corticosteroids, should be considered.
After asthma stability has been achieved, titrate to the lowest effective dosage to reduce the possibility of side effects.
3 DOSAGE FORMS AND STRENGTHS
Inhalation aerosol: Pressurized metered dose inhaler with a built in spacer that delivers 60 or 120 metered 80 mcg doses.
4 CONTRAINDICATIONS
AEROSPAN is contraindicated in the following conditions:
- Primary treatment of status asthmaticus or other acute episodes of asthma where intensive measures are required.
5 WARNINGS AND PRECAUTIONS
5.1 Local Infections
In clinical studies with flunisolide, localized infections with Candida albicans or Aspergillus niger have occurred in the mouth and pharynx and occasionally in the larynx. If oropharyngeal candidiasis develops, treat with appropriate local or systemic (i.e., oral) antifungal therapy while still continuing with AEROSPAN therapy, but at times therapy with AEROSPAN may need to be temporarily interrupted under close medical supervision. Rinsing the mouth after inhalation is advised. [see Adverse Reactions (6.1)].
5.2 Acute Asthma Episodes
AEROSPAN is not a bronchodilator and is not indicated for rapid relief of bronchospasm. Instruct patients to contact their physician immediately when episodes of asthma that are not responsive to bronchodilators occur during the course of treatment with AEROSPAN. During such episodes, patients may require therapy with systemic corticosteroids.
5.3 Immunosuppression
Patients who are using drugs that suppress the immune system are more susceptible to infections than healthy individuals. Chickenpox and measles, for example, can have a more serious or even fatal course in non-immune children or adults on corticosteroids. In such children or adults who have not had these diseases or been properly immunized, particular care should be taken to avoid exposure. How the dose, route, and duration of corticosteroid administration affects the risk of developing a disseminated infection is not known. The contribution of the underlying disease and prior corticosteroid treatment to the risk is also not known. If a patient is exposed to chickenpox, prophylaxis with varicella-zoster immune globulin (VZIG) may be indicated. If a patient is exposed to measles, prophylaxis with pooled intramuscular immunoglobulin (IG) may be indicated. (See the respective package inserts for complete VZIG and IG prescribing information.) If chickenpox develops, treatment with antiviral agents may be considered.
Because of the potential for worsening infections, use inhaled corticosteroids with caution, if at all, in patients with untreated active or quiescent tuberculosis infection of the respiratory tract; untreated systemic fungal, bacterial, parasitic or viral infections; or ocular herpes simplex.
5.4 Transfer from Systemic Corticosteroids
Particular care is needed in patients who are transferred from systemically active corticosteroids to AEROSPAN because deaths due to adrenal insufficiency have occurred in asthmatic patients during and after transfer from systemic corticosteroids to less systemically available inhaled corticosteroids. After withdrawal from systemic corticosteroids, a number of months are required for recovery of hypothalamic-pituitary-adrenal (HPA) function.
Patients who have been previously maintained on 20 mg or more per day of prednisone (or its equivalent) may be most susceptible, particularly when their systemic corticosteroids have been almost completely withdrawn. During this period of HPA suppression, patients may exhibit signs and symptoms of adrenal insufficiency when exposed to trauma, surgery or infections (particularly gastroenteritis) or other conditions associated with severe electrolyte loss. Although AEROSPAN may provide control of asthmatic symptoms during these episodes, in recommended doses it supplies less than the physiologic amounts of glucocorticoid (cortisol) systemically and does NOT provide the mineralocorticoid activity that is necessary for coping with these emergencies.
During periods of stress or a severe asthmatic attack, patients who have been withdrawn from systemic corticosteroids should be instructed to resume systemic steroids immediately and to contact their physician for further instruction. Instruct these patients to carry a warning card indicating that they may need supplementary systemic steroids during periods of stress or a severe asthma attack.
Wean patients requiring oral corticosteroids slowly from systemic corticosteroid use after transferring to AEROSPAN. Prednisone reduction can be accomplished by reducing the daily prednisone dose by 2.5 mg/day on a weekly basis [see Dosage and Administration (2)]. Lung function (forced expiratory volume in one second [FEV1] or morning peak expiratory flow rate [AM PEF]), beta-agonist use, and asthma symptoms should be carefully monitored during withdrawal of oral corticosteroids. In addition to monitoring asthma signs and symptoms, observe patients for signs and symptoms of adrenal insufficiency such as fatigue, lassitude, weakness, nausea and vomiting, and hypotension.
Transfer of patients from systemic corticosteroid therapy to AEROSPAN may unmask allergic conditions previously suppressed by the systemic corticosteroid therapy, e.g. rhinitis, conjunctivitis, eczema, arthritis, and eosinophilic conditions.
During withdrawal from oral corticosteroids, some patients may experience symptoms of systemically active corticosteroid withdrawal, e.g., joint or muscular pain, lassitude and depression, despite maintenance or even improvement of respiratory function.
5.5 Hypercorticism and Adrenal Suppression
In responsive patients, flunisolide may permit control of asthmatic symptoms with less suppression of HPA axis function than therapeutically equivalent oral doses of prednisone. Since flunisolide is absorbed into the circulation and can be systemically active, the beneficial effects of AEROSPAN in minimizing or preventing HPA axis dysfunction may be expected only when recommended dosages are not exceeded and individual patients are titrated to the lowest effective dose. Since individual sensitivity to effects on cortisol production exists, physicians should consider this information when prescribing AEROSPAN.
Because of the possibility of systemic absorption of inhaled corticosteroids, observe patients treated with AEROSPAN carefully for any evidence of systemic corticosteroid effects. Particular care should be taken in observing patients post-operatively or during periods of stress for evidence of inadequate adrenal response.
It is possible that systemic corticosteroid effects such as hypercorticism and adrenal suppression may appear in a small number of patients, particularly at higher doses. If such changes occur, reduce the AEROSPAN dose slowly, consistent with accepted procedures for management of asthma symptoms and for tapering of systemic corticosteroids.
5.6 Reduction in Bone Mineral Density
Decreases in bone mineral density (BMD) have been observed with long-term administration of products containing inhaled corticosteroids, including flunisolide. The clinical significance of small changes in BMD with regard to long-term outcomes is unknown. Monitor patients with major risk factors for decreased bone mineral content, such as prolonged immobilization, family history of osteoporosis, postmenopausal status, tobacco use, advanced age, poor nutrition, or chronic use of drugs that can reduce bone mass (e.g., anticonvulsants and corticosteroids) and treat with established standards of care.
5.7 Effects on Growth
Orally inhaled corticosteroids, including flunisolide, may cause a reduction in growth velocity when administered to pediatric patients [see Use in Specific Populations (8.4)]. Monitor the growth of children and adolescents receiving AEROSPAN. To minimize the systemic effects of orally inhaled corticosteroids, including AEROSPAN, titrate each patient to his/her lowest effective dose [see Dosage and Administration (2)].
5.8 Glaucoma and Cataracts
Glaucoma, increased intraocular pressure, and cataracts have been reported in patients following the long-term administration of inhaled corticosteroids, including flunisolide. Monitor patients closely, especially patients with a change in vision or with a history of increased intraocular pressure, glaucoma, or cataracts.
5.9 Paradoxical Bronchospasm
As with other inhaled asthma medications, bronchospasm may occur with an immediate increase in wheezing after dosing. If bronchospasm occurs following dosing with AEROSPAN, treat immediately with a fast-acting inhaled bronchodilator. Discontinue treatment with AEROSPAN immediately and institute alternative therapy.
6 ADVERSE REACTIONS
Systemic and local corticosteroid use may result in the following:
- Candida albicans infection [see Warnings and Precautions (5.1)]
- Immunosuppression, increased risk of infections [see Warnings and Precautions (5.3)]
- Hypercorticism and adrenal suppression [see Warnings and Precautions (5.5)]
- Reduction in bone mineral density [see Warnings and Precautions (5.6)]
- Effects on growth [see Warnings and Precautions (5.7)]
- Glaucoma, increased intraocular pressure and cataracts [see Warnings and Precautions (5.8)]
- Bronchospasm [see Warnings and Precautions (5.9)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Table 1 shows the adverse reactions that were reported in patients previously receiving bronchodilators and/or orally inhaled corticosteroids in two double-blind, placebo- controlled US clinical trials, in which 519 adult and pediatric patients age 4-78 years (279 males and 240 females) were treated with the AEROSPAN (80 mcg to 320 mcg twice daily for 12 weeks) or placebo. The mean duration of exposure was 76.7, 78.2, 80.5, and 69.4 days for AEROSPAN 80 mcg, 160 mcg, 320 mcg, and placebo, all dosed twice daily, respectively. The table includes all reactions that occurred at a rate of >3% in any AEROSPAN Inhalation Aerosol group. In considering these data, the increased average duration of exposure for AEROSPAN patients should be taken into account, compared with placebo-treated patients.
|
Table 1. Adverse Reactions with >3% incidence reported in controlled clinical studies with AEROSPAN (% of patients) |
||||
|
ADVERSE REACTION |
PLACEBO
|
AEROSPAN Inhalation Aerosol |
||
|
80 MCG
|
160 MCG
|
320 MCG
|
||
|
BODY AS A WHOLE | ||||
|
Headache |
12.7 |
9.0 |
13.8 |
8.8 |
|
Allergic Reaction |
2.3 |
4.2 |
4.6 |
4.4 |
|
Infection, Bacterial |
0.9 |
3.7 |
0.9 |
0.9 |
|
DIGESTIVE SYSTEM | ||||
|
Vomiting |
4.1 |
4.2 |
4.6 |
0.0 |
|
Dyspepsia |
1.4 |
2.1 |
3.2 |
3.5 |
|
RESPIRATORY SYSTEM | ||||
|
Pharyngitis |
13.2 |
17.5 |
16.6 |
16.8 |
|
Rhinitis |
10.0 |
9.0 |
15.7 |
3.5 |
|
Cough Increased |
7.7 |
8.5 |
5.5 |
1.8 |
|
Sinusitis |
5.5 |
7.4 |
4.1 |
8.8 |
|
Epistaxis |
0.9 |
3.2 |
0.9 |
0.0 |
|
UROGENITAL SYSTEM | ||||
|
Urinary Tract Infection |
0.5 |
1.1 |
0.9 |
3.5 |
The following other adverse reactions occurred in patients in these clinical trials using AEROSPAN with an incidence of 1 to 3% and were more common in AEROSPAN than in the placebo group.
Body As A Whole: abdominal pain, chest pain, infection, neck pain
Digestive System: diarrhea, gastroenteritis, nausea, oral moniliasis
Metabolic And Nutritional Disorders: edema
Musculoskeletal System: myalgia
Nervous System: dizziness, insomnia, migraine
Respiratory System: bronchitis, laryngitis, voice alteration
Skin And Appendages: erythema multiforme
Special Senses: conjunctivitis, ear pain, taste perversion
Urogenital System: dysmenorrhea, vaginitis
6.2 Adverse Reactions from Other Sources
The following additional adverse reactions were derived from clinical trials conducted with flunisolide CFC inhalation aerosol with a frequency of ≥1% and not described above:
Body as a Whole: flu, decreased appetite, chills, increased appetite, weight gain, malaise, peripheral edema, sweating, weakness
Gastrointestinal System: upset stomach, heartburn, constipation, gas, abdominal fullness
Cardiovascular System: palpitations, hypertension, tachycardia
Nervous System: headache, irritability, shakiness, anxiety, depression, faintness, fatigue, hyperactivity, hypoactivity, moodiness, numbness, vertigo
Respiratory System: cold symptoms, nasal congestion, upper respiratory tract infection, chest congestion, hoarseness, runny nose, sinus congestion, sinus drainage, sinus infection, sneezing, sputum, wheezing, chest tightness, bronchospasm, dyspnea, head stuffiness, nasal irritation, pleurisy, pneumonia, sinus discomfort
Skin and Appendages: eczema, pruritus, acne, urticaria
Special Senses: loss of smell, loss of taste, ear infection, blurred vision, eye discomfort, eye infection
Hemic and Lymph: capillary fragility, enlarged lymph nodes
Mouth and Throat: sore throat, dry throat, glossitis, mouth irritation, phlegm, throat irritation
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no studies with AEROSPAN in pregnant women to inform a drug‑associated risk for birth defects or miscarriage. There are clinical considerations with use of AEROSPAN in pregnant women to inform a drug-associated risk. [see Clinical Considerations.] In animal reproduction studies, flunisolide administered to pregnant rats and rabbits during the period of organogenesis produced fetal structural abnormalities. The flunisolide dose in rats and rabbits was approximately 3 and 1 times the maximum recommended human daily inhalation dose (MRHDID), respectively. [see Data.]
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
In two separate embryo-fetal developmental studies, pregnant rats and rabbits receiving flunisolide hemihydrate during the period of organogenesis at doses up to approximately 3 and 1 times, respectively, the MRHDID (on a mg/m2 basis at maternal doses of 200 and 40 mg/kg/day, respectively) showed structural abnormalities in their fetuses.
8.2 Lactation
Risk Summary
There is no information on the presence of flunisolide in human milk, or the effects on the breast fed child, or the effects on milk production following use of flunisolide. Because other corticosteroids are excreted in human milk, caution should be exercised when AEROSPAN is administered to nursing women.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for AEROSPAN and any potential adverse effects on the breastfed infant from AEROSPAN or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of AEROSPAN has been studied in children 4- 17 years of age. In clinical studies, the efficacy of AEROSPAN was not established in children 4-5 years of age, although the adverse reaction profile observed in patients exposed to AEROSPAN was similar between the 4-5 year age group (n=21), the 6-11 year age group (n=210), the 12-17 year age group (n=30), and those patients 18 years of age and older (n=258). The safety and effectiveness of AEROSPAN has not been studied in patients less than 4 years of age.
Effects on Growth
Controlled clinical studies have shown that orally inhaled corticosteroids may cause a reduction in growth velocity in pediatric patients. In these studies, the mean reduction in growth velocity was approximately one cm per year (range 0.3 to 1.8 cm per year) and appears to depend upon the dose and duration of exposure. This effect was observed in the absence of laboratory evidence of hypothalamic-pituitary-adrenal (HPA) axis suppression, suggesting that growth velocity is a more sensitive indicator of systemic corticosteroid exposure in pediatric patients than some commonly used tests of HPA axis function. The long-term effects of this reduction in growth velocity associated with orally inhaled corticosteroids, including the impact on final adult height, are unknown. The potential for "catch up" growth following discontinuation of treatment with orally inhaled corticosteroids has not been adequately studied. The growth of pediatric patients receiving orally inhaled corticosteroids, including AEROSPAN, should be monitored routinely (e.g., via stadiometry). The potential growth effects of prolonged treatment should be weighed against clinical benefits obtained and the risks/benefits of treatment alternatives. To minimize the systemic effects of orally inhaled corticosteroids, including AEROSPAN, each patient should be titrated to the lowest dose that effectively controls his/her symptoms.
The potential effect of AEROSPAN on growth rates in children was assessed in a 52 week randomized, placebo controlled study conducted in 242 prepubescent children age 4 to 9.5 years (145 males, 97 females) with mild persistent asthma. Treatment groups were AEROSPAN 160 mcg twice daily and placebo. Growth velocity was estimated for each patient using the slope of the linear regression of height over time using observed data in the intent to treat population who had at least 3 height measurements. The mean growth velocities were 6.19 cm/year in the placebo group and 6.01 cm/year in the AEROSPAN treated group (difference from placebo -0.17 cm/year; 95% CI: -0.58, 0.24).
8.5 Geriatric Use
Clinical studies of AEROSPAN included 21 patients 65 to 78 years of age exposed to AEROSPAN. These studies did not include sufficient numbers of patients aged 65 years and over to determine whether they respond differently from younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
10 OVERDOSAGE
In a double-blind, placebo-controlled study, 18 mg of flunisolide was administered via the CFC formulation over a three-hour period (nine times the maximum labeled daily dose) in 94 patients with acute asthma, and no clinically deleterious effects were observed.
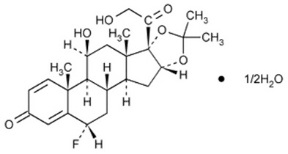
11 DESCRIPTION
Flunisolide, the active component of AEROSPAN® (flunisolide) inhalation aerosol, is a corticosteroid having the chemical name 6α-Fluoro-11β, 16α, 17, 21 –tetrahydroxylpregna-1, 4- diene-3, 20-dione cyclic-16, 17-acetal with acetone, hemihydrate and the following chemical structure:
Flunisolide is a white to creamy white crystalline powder with a molecular weight of 443.51 and an empirical formula of C24H31O6F ½ H2O. It is soluble in acetone, ethyl alcohol and HFA- 134a and practically insoluble in water.
AEROSPAN inhalation aerosol is a pressurized, metered-dose inhaler unit intended for oral inhalation only. The inhaler unit consists of a metal canister, a purple actuator, and a built-in gray spacer. Each unit contains a 0.24 % w/w solution of flunisolide in 10:90 w/w ethanol:1,1,1,2-tetrafluoroethane (HFA 134a). After priming, each actuation delivers 139 mcg of flunisolide in 58 mg of solution from the canister valve and 80 mcg of flunisolide (equivalent to 78 mcg flunisolide anhydrous) from the spacer at a flow rate of 30 L/min for 4 seconds.
Using an in-vitro method at a fixed volume of 2 L, each actuation at the beginning of canister content delivers from the spacer 76 mcg (95% of the label claim) at a flow rate of 30 L/min, 61 mcg (76% of the label claim) at a flow rate of 20 L/min, 85 mcg (106% of the label claim) at a flow rate of 40 L/min, and 96 mcg (120% of the label claim) at a flow rate of 60 L/min. The actual amount of drug delivered to the lung may depend upon patient factors, such as timing and coordination between the actuation and inspiration and the strength and duration of the inspiration. Data show that both the one- and two-actuation doses are depleted by 75% or more after waiting one second between actuation and inhalation.
The 5.1-g net weight canister provides 60 metered actuations and the 8.9-g net weight canister provides 120 metered actuations.
AEROSPAN should be primed before using for the first time by releasing 2 test sprays into the air away from the face. In cases where the inhaler has not been used for more than 2 weeks, the inhaler should be primed again by releasing 2 test sprays into the air away from the face.
This product contains a built-in spacer. Do not use with any external spacer devices.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Flunisolide has demonstrated marked anti-inflammatory activity in classical test systems. It is a corticosteroid that is several hundred times more potent than cortisol in animal anti-inflammatory assays, and several hundred times more potent than dexamethasone in anti-inflammatory effect as determined by the McKenzie skin blanching test. The clinical significance of these findings is unknown.
The precise mechanism of corticosteroid action is unknown. Airway inflammation is an important component in the pathogenesis of asthma. Corticosteroids have been shown to have a wide range of anti-inflammatory effects, inhibiting both inflammatory cells (e.g. mast cells, eosinophils, neutrophils, macrophages, and lymphocytes) and release of inflammatory mediators (e.g., histamine, eicosanoids, leukotrienes, and cytokines) involved in allergic and non-allergic-mediated inflammation. These anti-inflammatory actions of corticosteroids may contribute to their efficacy in asthma.
12.2 Pharmacodynamics
Dose finding for AEROSPAN was based on comparability of systemic exposure to flunisolide CFC inhalation aerosol. The effect of flunisolide CFC inhalation aerosol and AEROSPAN on pharmacokinetics and 12-hour plasma cortisol levels was investigated in two studies. In both studies, the Cmax and AUC of flunisolide, 6β-OH flunisolide, and 12-hour plasma cortisol measurements were comparable for 1000 mcg of flunisolide CFC inhalation aerosol and 320 mcg of AEROSPAN. The first was a parallel arm study in 31 subjects. Pharmacokinetics and plasma cortisol levels were determined after single and multiple doses of flunisolide CFC inhalation aerosol 1000 µg and AEROSPAN 160 µg or 320 µg administered twice daily for 13.5 days. At steady state, flunisolide mean peak plasma concentrations from flunisolide CFC inhalation aerosol 1000 mcg and AEROSPAN 320 mcg were found to be 2.6 ng/mL and 3.4 ng/mL, respectively. The corresponding mean AUC values for the 12-hr dosing interval were 5.7 ng.hr/mL and 4.7 ng.hr/mL, respectively. At steady state, the mean peak plasma concentrations of 6β-OH flunisolide from flunisolide CFC inhalation aerosol 1000 mcg and AEROSPAN 320 mcg were found to be 0.9 ng/mL and 0.3 ng/mL, respectively. The corresponding mean AUC values for the 12-hr dosing interval were 3.8 ng.hr/mL and 1.1 ng.hr/mL, respectively. The second was a crossover study in 11 subjects after single doses of flunisolide CFC inhalation aerosol 1000 mcg or AEROSPAN 320 mcg. The mean peak plasma concentrations of flunisolide from the flunisolide CFC inhalation aerosol 1000 mcg and AEROSPAN 320 mcg were found to be 2.5 ng/mL and 3.3 ng/mL, respectively. The corresponding mean AUC values were 5.1 ng.hr/mL and 5.8 ng.hr/mL, respectively. The mean peak plasma concentrations of 6 β -OH flunisolide from flunisolide CFC inhalation aerosol 1000 mcg and AEROSPAN 320 mcg were found to be 0.8 ng/mL and 0.3 ng/mL, respectively. The corresponding mean AUC values were 3.8 ng.hr/mL and 2.3 ng.hr/mL, respectively.
Controlled clinical studies with flunisolide CFC inhalation aerosol included over 500 treated asthma patients, among them 150 children aged 6 years and older. Open label studies of two years or more duration included more than 120 treated patients. No significant adrenal suppression attributed to flunisolide was seen in these studies.
The potential effects of AEROSPAN and flunisolide CFC inhalation aerosol on the hypothalamic-pituitary-adrenal (HPA) axis were studied in 2 placebo- and active- controlled studies and 2 active-controlled, open label, long-term studies [see Clinical Studies (14)]. In the placebo-controlled studies, the ability to increase cortisol production in response to stress was assessed by the 60 minute cosyntropin (ACTH) stimulation test. For adult and adolescent patients treated with AEROSPAN 80 mcg, 160 mcg, 320 mcg, or placebo twice daily for 12 weeks, 92% (22/24), 93% (26/28), 93% (26/28), and 92% (22/24) of patients, normal at baseline, respectively, continued to have a normal stimulated cortisol response (peak cortisol ≥18 mcg/dL and an increase in plasma cortisol ≥7 mcg/dL within 60 minutes after cosyntropin injection) at trial's end. All patients had peak cortisol levels ≥ 18mcg/dL. There was no significant suppression of 24 hour urinary cortisol, and 100% (96/96) of patients treated with AEROSPAN had normal morning serum cortisol levels at the end of study. For pediatric patients treated with the AEROSPAN, 80 mcg and 160 mcg or placebo twice daily for 12 weeks, 91% (31/34), 97% (28/29), and 89% (24/27) of patients, respectively, continued to have a normal stimulated cortisol response (as defined above) at trial's end. No suppression of 24-hour urinary cortisol was noted. In these studies, comparable results were obtained in patients treated with flunisolide CFC inhalation aerosol.
In the active-controlled, open label, long-term studies, 99.4% (161/162) of adult and adolescent patients and 98.4% (126/128) pediatric patients treated with AEROSPAN had normal morning serum cortisol levels (≥ 5 mcg/dL) after 12 or 52 weeks of treatment, respectively. For patients treated with AEROSPAN, 92.5% (99/107) continued to have a normal stimulated plasma cortisol response to cosyntropin at trial's end with all having peak cortisol levels ≥ 18mcg/dL. In these studies, no suppression of 24-hour urinary cortisol was noted, and comparable results were obtained in patients treated with flunisolide CFC inhalation aerosol.
12.3 Pharmacokinetics
All the data described below is based on studies conducted in subjects 18 to 51 years of age.
Absorption
Flunisolide is rapidly absorbed after oral inhalation. Mean values for the time to maximum concentration, Tmax, of flunisolide range from 0.09 to 0.17 hr after a single 320 mcg dose of AEROSPAN. The corresponding mean values for the maximum concentration, Cmax, of flunisolide vary from 1.9 to 3.3 ng/mL. Oral bioavailability is less than 7%. Over the dose range of 80 mcg to 320 mcg of AEROSPAN, values for Cmax increase proportionately with dose after single as well as multiple dose administration.
Distribution
Flunisolide is extensively distributed in the body, with mean values for apparent volume of distribution ranging from 170 to 350 L after a single 320 mcg dose of AEROSPAN.
Metabolism
Flunisolide is rapidly and extensively converted to 6ß-OH flunisolide and to water-soluble conjugates during the first pass through the liver. Conversion to 6ß-OH flunisolide, the only circulating metabolite detected in man, is thought to occur via the cytochrome P450 enzyme system, particularly the enzyme CYP3A4. 6ß-OH flunisolide has a low corticosteroid potency (ten times less potent than cortisol and more than 200 times less potent than flunisolide). Maximum levels of 6ß-OH flunisolide were 0.66 mcg/mL after a single 320 mcg dose of AEROSPAN, and 0.71 mcg/mL after multiple doses of AEROSPAN.
Excretion
Urinary excretion of flunisolide is low. Less than 1% of the administered dose of flunisolide is recovered in urine after inhalation. The half-life values for 6ß-OH flunisolide range from 3.1 to 5.1 hrs after administration of AEROSPAN in the dose range of 160 mcg to 320 mcg.
Disposition and Elimination
Twice daily inhalation administration of flunisolide for up to 14 days did not result in appreciable accumulation of flunisolide. Upon multiple dosing with 160 mcg and 320 mcg, the Cmax values were 1.0 ng/mL and 2.1 ng/mL, respectively. The corresponding AUC0-12hr values were 1.2 ng.hr/mL and 2.5 ng.hr/mL.
Flunisolide is rapidly cleared from the body, independent of route of administration or dose administered. Flunisolide is not detectable in plasma twelve hours post-dose.
After administration of 320 mcg of AEROSPAN the elimination half-life ranges from 1.3 to 1.7 hours. After a single 320 mcg dose, mean oral clearance values, not adjusted for bioavailability, range from 83 to 167 L/hr.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 22 month study in Swiss mice, flunisolide at oral doses up to 500 mcg/kg/day (approximately 3 and 4 times the maximum recommended daily inhalation dose [MRDID] in adults and children on a mg/m2 basis) did not demonstrate any carcinogenic effects.
In a two year study in Sprague Dawley rats, administration of flunisolide in the diet at a dose of 2.5 mcg/kg/day (less than MRDID in adults or children on a mg/m2 basis) resulted in an increased incidence of mammary gland adenomas and islet cell adenomas of the pancreas in females. The significance of these findings for humans is unknown. There were no significant increases in the incidence of any tumor type in female rats at a dose of 1.0 mcg/kg/day (less than MRDID in adults or children on a mg/m2 basis), or in male rats at a dose of 2.5 mcg/kg/day (less than MRDID in adults or children on a mg/m2 basis).
Flunisolide showed no mutagenic activity when tested in in vitro bacterial assay systems (Ames Assay and the Rec-assay) and no clastogenic activity when tested in the in vitro chromosomal aberration assay using Chinese Hamster CHL cells and in the in vivo mouse bone marrow chromosomal aberration assay.
Studies on the effects of flunisolide on fertility in female rats showed that flunisolide, at an oral dose of 200 mcg/kg/day (approximately 3 times MRDID on a mg/m2 basis) impaired fertility, but was devoid of such effects at doses up to 40 mcg/kg/day (less than MRDID on a mg/m2 basis).
14 CLINICAL STUDIES
The efficacy of AEROSPAN has been studied in two double-blind, parallel, placebo-and active-controlled clinical trials of 12 weeks duration involving more than 1250 patients, one in adults and adolescents 12 years of age and older, and one in patients 4-11 years of age. In adults and adolescents, efficacy was evaluated in patients previously treated with inhaled corticosteroids. In children 6 to 11 years of age, efficacy was evaluated in patients previously treated with bronchodilators alone or inhaled corticosteroids. Both trials had a 2- week run-in period followed by a 12-week randomized treatment period. During the run-in period all patients received flunisolide CFC inhalation aerosol 500 mcg twice daily. Patients were then randomized to double-blind treatment with different doses of AEROSPAN or flunisolide CFC inhalation aerosol and monitored for lung function changes to see if they maintained, improved, or lost stability. Baseline was assessed at the end of the run-in period. The primary endpoint was the change from baseline in percent predicted FEV1 after 12 weeks treatment.
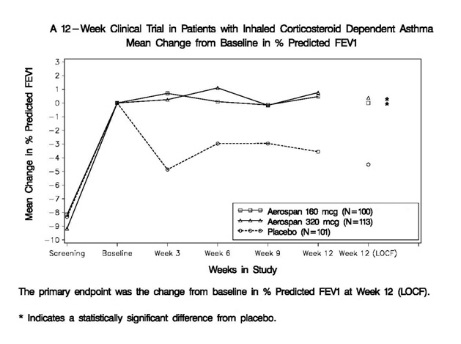
14.1 Adult and Adolescent Patients 12 Years of Age and Older
Efficacy was evaluated in 669 asthma patients, 12 to 78 years of age, including 88 patients 12-17 years of age and 581 patients 18 years and older. Mean FEV1 at screening was 2.44 L and mean FEV1 at baseline was 2.72 L following the 2-week run-in period. Patients were randomized to AEROSPAN 80 mcg, 160 mcg or 320 mcg twice daily, flunisolide CFC inhalation aerosol 250 mcg, 500 mcg, or 1000 mcg twice daily, or placebo. Change from baseline in percent predicted FEV1 over 12 weeks treatment demonstrated that placebo patients deteriorated 4.3% from baseline after 12 weeks of treatment, whereas patients treated with AEROSPAN 160 mcg or 320 mcg twice daily maintained FEV1 over the course of the study. Results for the comparison to placebo were statistically significant for the 160 and 320 mcg twice daily AEROSPAN doses (see Figure 1), but not for the 80 mcg dose. Secondary endpoints of AM peak expiratory flow rate, AM and PM asthma symptoms, nocturnal awakenings requiring a β2 agonist, and as needed use of inhaled β2 agonists showed differences from baseline favoring AEROSPAN over placebo. AEROSPAN and flunisolide CFC inhalation aerosol gave comparable results.
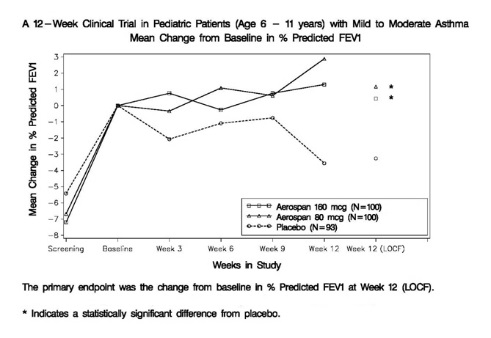
14.2 Pediatric Patients 4 to 11 Years of Age
The trial enrolled 583 asthma patients, 4 to 11 years of age, although the primary efficacy parameter was evaluated only in the population of 513 patients 6 to 11 years of age. In these patients, the mean FEV1 at screening was 81.2% predicted, and the mean FEV1 at baseline following a two week run-in period was 87.5% predicted. Patients were randomized to AEROSPAN 80 mcg or 160 mcg twice daily, flunisolide CFC inhalation aerosol 250 mcg or 500 mcg twice daily, or placebo. Change from baseline in percent predicted FEV1 over 12 weeks in patients 6 years of age and older demonstrated that placebo patients deteriorated 4.0% from baseline after 12 weeks of treatment, whereas patients treated with AEROSPAN 80 mcg or 160 mcg twice daily maintained FEV1 over the course of the study. Results for the comparison to placebo were statistically significant for the 80 mcg and 160 mcg doses of AEROSPAN, but there was no added benefit for the 160 mcg BID dose over the 80 mcg BID dose (see Figure 2). AEROSPAN and flunisolide CFC inhalation aerosol gave comparable results in patients 6 years of age and older.
16 HOW SUPPLIED/STORAGE AND HANDLING
AEROSPAN is supplied as a pressurized lined aluminum canister in boxes of one. Each canister is supplied with a two-piece plastic purple actuator and gray spacer assembly, and tear-off patient's instructions, including a Patient Information and an illustrated. Instructions for Using Your AEROSPAN.
The following canister sizes are available: 8.9 g net weight, providing 120 metered actuations (trade size, NDC: 0037-7590-12); 5.1 g net weight providing 60 metered actuations (professional sample, NDC: 0037-7590-06); 5.1 g net weight providing 60 metered actuations (institutional pack, NDC: 0037-7590-63).
Prime the inhaler by releasing two test sprays into the air away from the face before first use of AEROSPAN, and when the inhaler has not been used for more than 2 weeks.
Instruct patients to prepare the inhaler for use by pulling the built-in purple actuator out from the gray spacer and snapping into an "L" shape prior to use.
The appearance of a white ring on the orifice of the actuator and inside the spacer is normal. The performance of AEROSPAN is not affected by this residue. No cleaning is required.
When not in use, keep AEROSPAN out of reach of children. Pediatric patients should only administer AEROSPAN under adult supervision.
The plastic purple actuator and gray spacer assembly supplied as part of AEROSPAN should not be used with any other product canisters; and the actuator from other products should not be used with an AEROSPAN canister. Do not separate the purple actuator from the gray spacer. Do not use this product with any external spacer or holding chamber devices.
The labeled amount of medication in each actuation (80 mcg flunisolide) cannot be assured after 120 metered actuations (or 60 metered actuations in the hospital and sample size canisters), even though the canister is not completely empty and will continue to operate. The inhaler (canister plus actuator) should be discarded when the labeled number of actuations have been used.
Avoid spraying in eyes.
Contents under pressure: Do not puncture. Do not use or store near heat or open flame. Protect from freezing temperatures and prolonged exposure to sunlight. Exposure to temperatures above 120°F (49°C) may cause bursting. Never throw into fire or incinerator.
17 PATIENT COUNSELING INFORMATION
See FDA-approved Patient Labeling (Patient Information and Instructions for Use).
Oral Candidiasis
Advise patients that localized fungal infections occurred in the mouth and pharynx in some patients. If oropharyngeal candidiasis develops, treat with appropriate local or systemic (i.e., oral) antifungal therapy while still continuing with AEROSPAN therapy, but at times therapy with AEROSPAN may need to be temporarily interrupted under close medical supervision. Rinsing the mouth after inhalation is advised.
Status Asthmaticus and Acute Asthma Symptoms
Advise patients that AEROSPAN is not a bronchodilator and is not intended to be used to treat status asthmaticus or to relieve acute asthma symptoms. Treat acute asthma symptoms with an inhaled, short-acting beta-2 agonist such as albuterol. Instruct patients to contact their physicians immediately if there is deterioration of their asthma.
Immunosuppression
Warn patients who are on immunosuppressant doses of AEROSPAN to avoid exposure to chickenpox or measles and, if exposed, to consult their physician without delay. Inform patients of potential worsening of existing tuberculosis, fungal, bacterial, viral, or parasitic infections, or ocular herpes simplex.
Hypercorticism and Adrenal Supression
Advise patients that AEROSPAN may cause systemic corticosteroid effects of hypercorticism and adrenal suppression. Additionally, instruct patients that deaths due to adrenal insufficiency have occurred during and after transfer from systemic corticosteroids. Taper patients slowly from systemic corticosteroids if transferring to AEROSPAN.
Reduction in Bone Mineral Density
Advise patients who are at an increased risk for decreased BMD that the use of corticosteroids may pose an additional risk. Monitor patients and, where appropriate, treat for this condition.
Reduced Growth Velocity
Inform patients that orally inhaled corticosteroids, including AEROSPAN, may cause a reduction in growth velocity when administered to pediatric patients. Physicians should closely follow the growth of children and adolescents taking corticosteroids by any route.
Ocular Effects
Long-term use of inhaled corticosteroids, including AEROSPAN, may increase the risk of some eye problems (cataracts or glaucoma); consider regular eye examinations.
Use Daily for Best Effect
Advise patients to use AEROSPAN at regular intervals as directed, since its effectiveness depends on regular use. Individual patients will experience a variable time to onset and degree of symptom relief, and the full benefit may not be achieved until treatment has been administered for 2 to 4 weeks. If symptoms do not improve in that time frame or if the condition worsens, patients should not increase dosage, but should contact the physician immediately.
Advise patients not to stop Aerospan or change the dose without talking with a healthcare provider. Advise patients that if they miss a dose to take the next scheduled dose when it is due.
Instructions for Use
Aerospan contains a built-in spacer. Do not use with any external spacer or holding chamber devices. Instruct patients to prepare the inhaler for use by pulling the built-in purple actuator out from the gray spacer and snapping into an "L" shape prior to use.
With use, the appearance of a white ring on the orifice of the actuator and inside the spacer is normal. The performance of AEROSPAN is not affected by this residue. No cleaning is required.
Distributed by:
Meda Pharmaceuticals Inc.
Somerset, New Jersey 08873-4120
©2018 Meda Pharmaceuticals Inc.
AEROSPAN is a registered trademark used under license by Meda Pharmaceuticals Inc.
MEDA and the MEDA PHARMACEUTICALS logo are registered trademarks of Meda AB
U.S. Patent Nos. 5,776,433; 6,230,704
Patient Information
AEROSPAN® (AIR-OH-SPAN)
(flunisolide)
Inhalation Aerosol
Note: For Oral Inhalation Only
Read this Patient Information leaflet before you start using AEROSPAN Inhalation Aerosol and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or your treatment. If you have any questions about AEROSPAN Inhalation Aerosol, ask your healthcare provider or pharmacist.
What is AEROSPAN Inhalation Aerosol?
AEROSPAN Inhalation Aerosol is a prescription medicine used for the long-term (maintenance) treatment of asthma to control and prevent asthma symptoms in adults and children 6 years of age and older.
AEROSPAN Inhalation Aerosol contains flunisolide, which is a man-made (synthetic) corticosteroid. Corticosteroids are natural substances found in the body. Inhaled corticosteroids help reduce airway inflammation that can lead to asthma symptoms.
When inhaled regularly, AEROSPAN Inhalation Aerosol will help to prevent and control asthma symptoms.
AEROSPAN Inhalation Aerosol is not a bronchodilator and does not treat sudden symptoms of an asthma attack, such as wheezing, cough, shortness of breath, and chest pain or tightness. Always use a fast-acting bronchodilator medicine (rescue inhaler), such as albuterol, to treat sudden symptoms.
Who should not use AEROSPAN Inhalation Aerosol?
Do not use AEROSPAN Inhalation Aerosol:
- to treat the symptoms of a sudden asthma attack or status asthmaticus.
- if you are allergic to flunisolide or any of the ingredients in AEROSPAN Inhalation Aerosol. See the end of this Patient Information leaflet for a complete list of ingredients in AEROSPAN Inhalation Aerosol.
AEROSPAN Inhalation Aerosol is not for use in children less than 6 years of age.
What should I tell my healthcare provider before using AEROSPAN Inhalation Aerosol?
Before you use AEROSPAN Inhalation Aerosol tell your healthcare provider if you:
- have or have had eye problems such as increased intraocular pressure, glaucoma, or cataracts.
- have any infections.
- have or had tuberculosis or herpes simplex of the eye.
- have not had or been vaccinated for chicken pox or measles, or have recently been near anyone with chicken pox or measles.
- are planning on having surgery.
- are pregnant or plan to become pregnant. It is not known if AEROSPAN Inhalation Aerosol will harm your unborn baby. Talk to your healthcare provider if you are pregnant or plant to become pregnant.
- are breastfeeding or plan to breastfeed. It is not known if AEROSPAN Inhalation Aerosol passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you are using AEROSPAN Inhalation Aerosol.
Tell your healthcare provider about all medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
Know all the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I use AEROSPAN Inhalation Aerosol?
- Read the Instructions for Use at the end of this leaflet for specific information about the right way to use AEROSPAN Inhalation Aerosol.
- Use AEROSPAN Inhalation Aerosol exactly as your healthcare provider tells you to use it. Do not take more of your medicine, or take it more often than your healthcare provider tells you.
- You must use your AEROSPAN Inhalation Aerosol regularly. It may take 2 weeks or longer after you start using AEROSPAN Inhalation Aerosol for your asthma symptoms to get better. Call your healthcare provider right away if your breathing problems do not improve or get worse. Call your healthcare provider if your symptoms do not improve after 4 weeks. Do not stop using AEROSPAN Inhalation Aerosol, and do not change the amount of AEROSPAN Inhalation Aerosol you take, without talking to your doctor. If you miss a dose, just take your next scheduled dose when it is due.
- An adult should help a child use their AEROSPAN Inhalation Aerosol.
What are the possible side effects of AEROSPAN Inhalation Aerosol?
AEROSPAN Inhalation Aerosol may cause serious side effects, including:
- fungal infections (thrush) in your mouth or throat. Tell your healthcare provider if you have any redness or white colored patches in your mouth or throat. Rinse your mouth after you use your AEROSPAN Inhalation Aerosol.
-
immune system problems that may increase your risk of infections. You are more likely to get infections if you take medicines that may weaken your immune system. Avoid contact with people who have contagious diseases such as chicken pox or measles while you use AEROSPAN Inhalation Aerosol. Symptoms of an infection may include:
- fever
- pain
- aches
- chills
- feeling tired
- nausea
- vomiting
- Tell your healthcare provider about any signs of infection while you are using AEROSPAN Inhalation Aerosol.
-
decreased adrenal function (adrenal insufficiency). Adrenal insufficiency is a condition in which the adrenal glands do not make enough steroid hormones. Symptoms of adrenal insufficiency include:
- tiredness
- weakness
- dizziness
- nausea
- vomiting
- decreased bone mass (bone mineral density). People who use inhaled steroid medicines for a long time may have an increased risk of decreased bone mass which can affect bone strength. Talk to your healthcare provider about any concerns you may have about bone health.
- slowed or delayed growth in children. A child's growth should be checked regularly while taking AEROSPAN Inhalation Aerosol.
- eye problems such as glaucoma and cataracts. If you have a history of glaucoma or cataracts or have a family history of eye problems, you should have regular eye exams while you use AEROSPAN Inhalation Aerosol.
- increased wheezing (bronchospasm) can happen right away after using AEROSPAN Inhalation Aerosol. Stop using AEROSPAN Inhalation Aerosol and use an inhaled fast-acting bronchodilator (rescue inhaler) right away.
The most common side effects with AEROSPAN Inhalation Aerosol include:
- sore throat (pharyngitis)
- runny nose (rhinitis)
- headache
- sinusitis
- increased cough
Tell your healthcare provider about any side effect that bothers you or that does not go away.
These are not all of the possible side effects of AEROSPAN Inhalation Aerosol. For more information, ask your doctor or pharmacist.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store AEROSPAN Inhalation Aerosol?
- Store AEROSPAN Inhalation Aerosol at room temperature between 59°F and 86°F (15°C to 30°C).
- Do not puncture the AEROSPAN Inhalation Aerosol canister.
- Do not store the AEROSPAN Inhalation Aerosol canister near heat or a flame. Exposure to temperature above 120°F (49°C) may cause the canister to burst.
- Do not throw the AEROSPAN Inhalation Aerosol canister into a fire or an incinerator.
- Do not freeze AEROSPAN Inhalation Aerosol.
- Safely throw away medicine that is out of date or no longer needed.
Keep AEROSPAN Inhalation Aerosol and all medicines out of the reach of children.
General information about the safe and effective use of AEROSPAN Inhalation Aerosol.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information Leaflet. Do not use AEROSPAN Inhalation Aerosol for a condition for which it was not prescribed. Do not give AEROSPAN Inhalation Aerosol to other people, even if they have the same symptoms that you have. It may harm them.
This Patient Information summarizes the most important information about AEROSPAN Inhalation Aerosol. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about AEROSPAN Inhalation Aerosol that is written for health professionals.
For more information, go to www.aerospan.net
What are the ingredients in AEROSPAN Inhalation Aerosol?
Active ingredient: Flunisolide
Inactive ingredients: propellant HFA-134a and ethanol
Instructions for Use
AEROSPAN® (AIR-OH-SPAN)
(flunisolide)
inhalation aerosol, for oral inhalation use
Read this Instructions for Use for AEROSPAN inhalation aerosol before you start using it and each time you get a refill. There may be new information. This leaflet does not take the place of talking to your doctor about your medical condition or treatment.
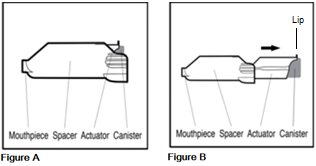
The parts of your AEROSPAN inhalation aerosol
AEROSPAN inhalation aerosol is an inhaler unit made up of 3 parts, the canister, actuator, and spacer. The purple inner part is called the actuator. It holds the metal canister of medicine. The gray outer part is called the spacer. It includes the mouthpiece through which you inhale the medicine (See Figure A and Figure B).
- Do not separate the purple actuator from the gray spacer.
- Do not use your AEROSPAN inhalation aerosol purple actuator and gray spacer with any other canisters.
- Do not use actuators from other medicines with your AEROSPAN inhalation aerosol canister.
- Do not use your AEROSPAN inhalation aerosol with any other spacers or holding chamber devices.
Using your AEROSPAN inhalation aerosol
Step 1. Your AEROSPAN inhalation aerosol needs to be opened and positioned before you can use it.
- Pull the purple actuator out from the gray spacer. You will see that there are ribbed finger pads on the sides of the gray spacer.
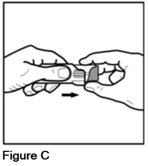
- Grip the ribbed finger pads on the sides of the gray spacer with the thumb and index finger of 1 hand (See Figure C).
- Gently pull on the lip at the top of the purple actuator with the other hand (See Figure D).
- Check the spacer and mouthpiece for foreign objects.
-
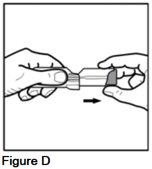
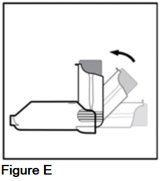
Snap your AEROSPAN inhalation aerosol into an "L" shape (90° angle) (See Figure E).
Your AEROSPAN inhalation aerosol will only move in 1 direction.
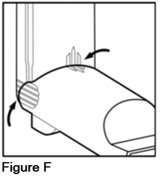
- Check to make sure that the guide lines on the purple actuator and the gray spacer match up (See Figure F).
- The lines on the ribbed finger pads of the gray spacer should match up with the lines across the back of the purple actuator.
- The lines on the top of the gray spacer and the purple actuator should match up. Make sure there is no opening at the top of the unit where the purple actuator and the gray spacer come together.
- Your AEROSPAN inhalation aerosol is ready for use when the lines are matched up.
Step 2. Prepare (prime) your AEROSPAN inhalation aerosol for use.
Note: If you have already primed your AEROSPAN inhalation aerosol, go to Step 3.
- Before you use your AEROSPAN inhalation aerosol for the first time or if you have not used your medicine for more than 2 weeks, you will need to prepare (prime) your AEROSPAN inhalation aerosol.
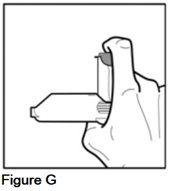
- Place the bottom of the gray spacer on the base of your thumb, and your index finger on the top of the metal canister. Your hand will be at the side of the device (See Figure G).
- Check to make sure that the metal canister is placed fully into the purple actuator.
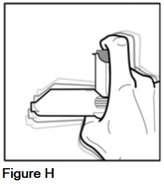
- Shake the inhaler (See Figure H).
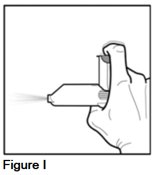
- To avoid spraying medicine in your eyes, point the mouthpiece away from your face.
- Press down on the metal canister 2 times, for 1 second each time, in order to release 2 test sprays into the air (See Figure I).
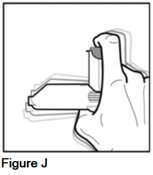
Step 3. Hold your AEROSPAN inhalation aerosol between your thumb and index finger, and shake the inhaler (See Figure J).
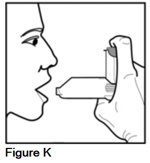
Step 4. Stand upright with your neck and head straight. Hold your AEROSPAN inhalation aerosol with the metal canister facing up and the mouthpiece of the spacer facing toward you. Place your thumb on the bottom of the gray spacer and your index finger on the top of the metal canister (See Figure K).
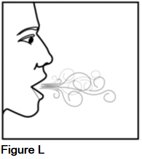
Step 5. Breathe in (inhale) and breathe out (exhale) normally through your mouth (See Figure L).
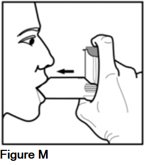
Step 6. After breathing out, bring your AEROSPAN inhalation aerosol to your mouth and hold the mouthpiece firmly between your lips (See Figure M).
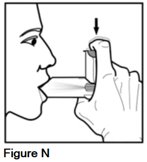
Step 7. Start breathing in slowly. While breathing in, fully depress the metal canister with your index finger for at least 1 second. Continue to breathe in through your mouth for 3 more seconds (See Figure N).
It is important to begin breathing in through your mouth right before pressing down on the metal canister. This helps you get the right amount of medicine.
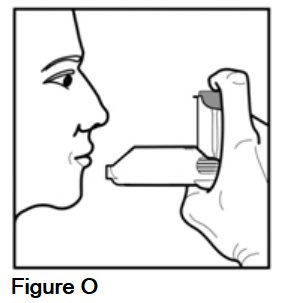
Step 8. After 3 seconds, remove your AEROSPAN inhalation aerosol from your mouth but do not breathe out yet. Close your lips and hold your breath for at least 10 seconds or as long as you feel comfortable (See Figure O).
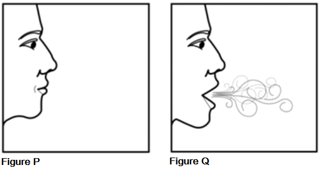
Step 9. After 10 seconds of holding your breath (See Figure P), breathe out and then breathe normally (See Figure Q).
Note: If your doctor has prescribed 2 or more sprays (inhalations) at each use, wait 20 seconds and then repeat Steps 3 to 9.
Closing your AEROSPAN inhalation aerosol
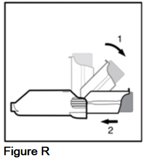
- Snap the purple actuator back to the straight position (See Figure R).
- Gently push the purple actuator back into the gray spacer (See Figure R).
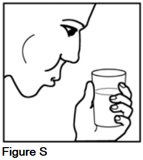
Rinse your mouth thoroughly with water and spit it out. This removes any remaining medicine from your mouth. You may also want to brush your teeth (See Figure S).
Note: If your mouth becomes sore or you get a yeast infection in your mouth (thrush), tell your doctor but do not stop using your inhaler unless your doctor tells you to stop.
Replacing your AEROSPAN inhalation aerosol
- Use a Check-Off chart to keep track of the amount of medicine you have used. Shaking the canister will not tell you how much medicine is left in your AEROSPAN inhalation aerosol.
- If your canister has 60 sprays, use the AEROSPAN inhalation aerosol 60 Sprays Check-Off chart.
- If your canister has 120 sprays, use the AEROSPAN inhalation aerosol 120 Sprays Check-Off chart.
- You will receive a new AEROSPAN inhalation aerosol each time you refill your prescription. Throw away your old AEROSPAN inhalation aerosol when you refill your prescription.
AEROSPAN inhalation aerosol 60 Sprays Check-Off Chart
- Keep this Check-Off chart with your AEROSPAN inhalation aerosol.
- If you are using the inhaler for the first time or if you have not used your inhaler for more than 2 weeks you will need to prepare (prime) your AEROSPAN inhalation aerosol. Follow the instructions provided above in “Step 2. Prepare (prime) your AEROSPAN inhalation aerosol for use.”
- Check off the two circled Ps (P = prime) as shown in the chart below (See Figure 1).
- Check off a numbered circle each time you use your inhaler. Count each spray. Start with spray number 60.
- Before you reach the last number of sprays, contact your doctor to find out if you need a refill.
- Throw away your AEROSPAN inhalation aerosol, which includes the canister, actuator, and spacer, after 60 sprays even if the canister is not empty. You may not get the right amount of medicine in each spray if you use your AEROSPAN inhalation aerosol after 60 sprays.
- Never place the canister under water to find out the amount of medicine still left in the canister ("float test").
AEROSPAN inhalation aerosol 120 Sprays Check-Off Chart
- Keep this Check-Off chart with your AEROSPAN inhalation aerosol.
- If you are using the inhaler for the first time or if you have not used your inhaler for more than 2 weeks you will need to prepare (prime) your AEROSPAN inhalation aerosol. Follow the instructions provided above in “Step 2. Prepare (prime) your AEROSPAN inhalation aerosol for use.”
- Check off the two circled Ps (P = prime) in the chart below (See Figure 2).
- Check off a numbered circle each time you use your inhaler. Count each spray. Start with spray number 120.
- Before you reach the last number of sprays, contact your doctor to find out if you need a refill.
- Throw away your AEROSPAN inhalation aerosol, which includes the canister, actuator, and spacer, after 120 sprays have been used even if the canister is not empty. You may not get the right amount of medicine in each spray if you use your AEROSPAN inhalation aerosol after 120 sprays.
- Never place the canister under water to find out the amount of medicine still left in the canister ("float test").
This Patient Information and Instructions for Use have been approved by the U.S. Food and Drug Administration.
Distributed by:
Meda Pharmaceuticals Inc.
Somerset, New Jersey 08873-4120
©2018 Meda Pharmaceuticals Inc.
AREOSPAN is a registered trademark used under license by Meda Pharmaceuticals Inc.
MEDA and the MEDA PHARMACEUTICALS logo are registered trademarks
of Meda AB
U.S. Patent Nos. 5,776,433; 6,230,704
IN-759006-06 Rev. 12/2017
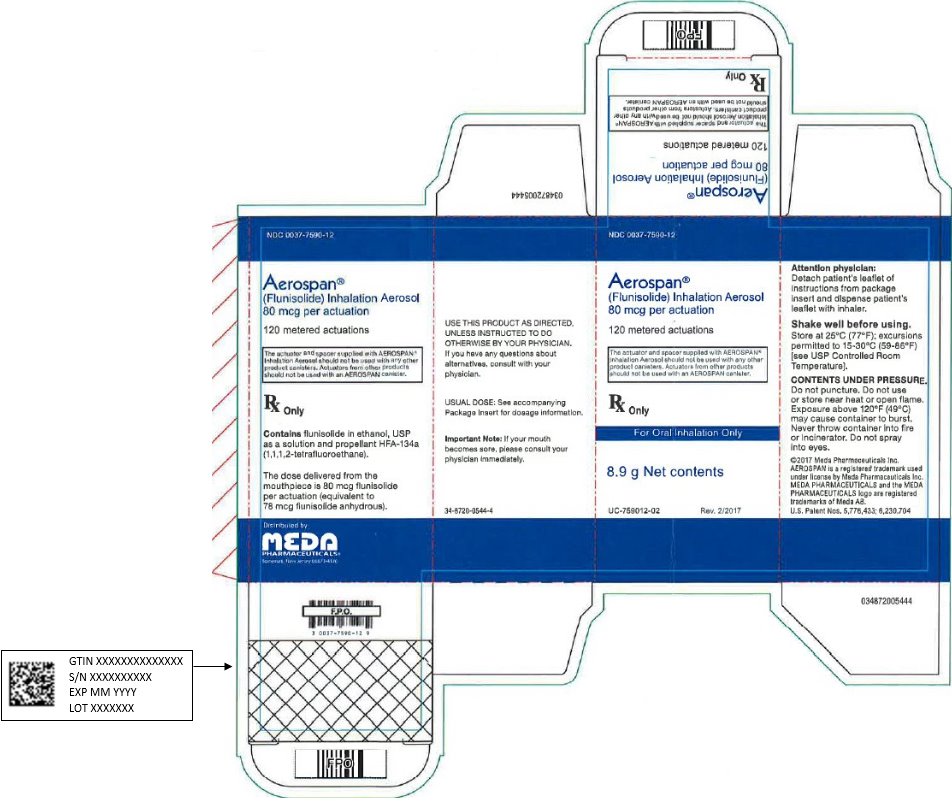
PRINCIPAL DISPLAY PANEL – 80 mcg per Actuation
NDC: 0037-7590-12
Aerospan®
(Flunisolide) Inhalation Aerosol
80 mcg per actuation
120 metered actuations
The actuator and spacer supplied with AEROSPAN®
Inhalation Aerosol should not be used with any other
product canisters. Actuators from other products
should not be used with an AEROSPAN canister.
Rx Only
For Oral Inhalation Only
8.9 g Net contents
UC-759012-02 Rev. 2/2017
Attention physician:
Detach patient’s leaflet of
instructions from package
insert and dispense patient’s
leaflet with inhaler.
Shake well before using.
Store at 25°C (77°F); excursions
permitted to 15-30°C (59-86°F)
[see USP Controlled Room
Temperature].
CONTENTS UNDER PRESSURE.
Do not puncture. Do not use
or store near heat or open flame.
Exposure above 120°F (49°C)
may cause container to burst.
Never throw container into fire
or incinerator. Do not spray
into eyes.
©2017 Meda Pharmaceuticals Inc.
AEROSPAN is a registered trademark used
under license by Meda Pharmaceuticals Inc.
MEDA PHARMACEUTICALS and the MEDA
PHARMACEUTICALS logo are registered
trademarks of Meda AB.
U.S. Patent Nos. 5,776,433; 6,230,704
USE THIS PRODUCT AS DIRECTED.
UNLESS INSTRUCTED TO DO
OTHERWISE BY YOUR PHYSICIAN.
If you have any questions about
alternatives, consult with your
physician.
USUAL DOSE: See accompanying
Package Insert for dosage information.
Important Note: If your mouth
becomes sore, please consult your
physician immediately.
34-8720-0544-4
Contains flunisolide in ethanol, USP
as a solution and propellant HFA-134a
(1,1,1,2-tetrafluoroethane).
The dose delivered from the
mouthpiece is 80 mcg flunisolide
per actuation (equivalent to
78 mcg flunisolide anhydrous).
Distributed by:
Meda Pharmaceuticals Inc.
Somerset, New Jersey 08873-4120
| AEROSPAN
flunisolide aerosol, metered |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Meda Pharmaceuticals (051229602) |