Dolodent by Pharmadel LLC Dolodent (HCare)
Dolodent by
Drug Labeling and Warnings
Dolodent by is a Homeopathic medication manufactured, distributed, or labeled by Pharmadel LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
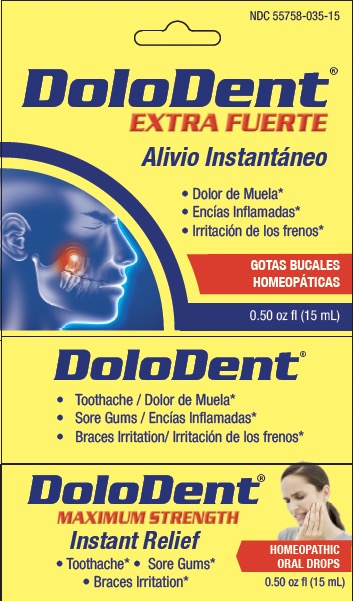
DOLODENT- arnica montana, chamomilla, hecla lava, hypericum, asafoedida liquid
Pharmadel LLC
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Dolodent (HCare)
Active ingredients & Purposes
| Active ingredients** (HPUS) | Purposes* |
| Arnica montana 30X | Pain in the tooth, with inflammation |
| Asafoetida 6X | Cavity pain |
| Chamomilla vulgaris 30X | Irritation, toothache, sore gums |
| Hekla lava 30X | Tooth sensitive to pressure, with swollen gums |
| Hypericum perforatum 30X |
Sharp shooting pain, due to toothache |
**The letters HPUS indicate that this ingredient is officially included in the Homeopathic Pharmacopeia of the United States.
Warnings
Directions
- shake bottle well use
- adult and children 12 years of age and over:
- add 2-3 drops to a small piece of cotton or gauze
- apply to the affected area for about 1 minute
- repeat as needed
- children under 12 years of age: consult the advice and supervision of a dentist or doctor
Distributed by:/ Distribuido por:
PHARMADEL
New Castle, DE, 19720
+ 1-866-359-3478
www.pharmadel.com
FB/ PHARMADELUSA
| DOLODENT
arnica montana, chamomilla, hecla lava, hypericum, asafoedida liquid |
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
| Labeler - Pharmadel LLC (030129680) |
Revised: 9/2023
Document Id: 0647abcf-743b-cbed-e063-6294a90ad272
Set id: ac3af7cf-e379-32c9-e053-2a95a90abae8
Version: 10
Effective Time: 20230926
Trademark Results [Dolodent]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 DOLODENT 86500592 4909897 Live/Registered |
Pharmadel Llc 2015-01-12 |
 DOLODENT 72275477 0853828 Dead/Expired |
STUR-DEE HEALTH PRODUCTS, INC. 1967-07-06 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.