Capsaicin by Skya Health, LLC Capsaicin Patch
Capsaicin by
Drug Labeling and Warnings
Capsaicin by is a Otc medication manufactured, distributed, or labeled by Skya Health, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CAPSAICIN- capsaicin patch
Skya Health, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Capsaicin Patch
CAPSAICIN – Capsaicin 0.025% Patch
Skya Health, LLC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Capsaicin 0.025% Cream
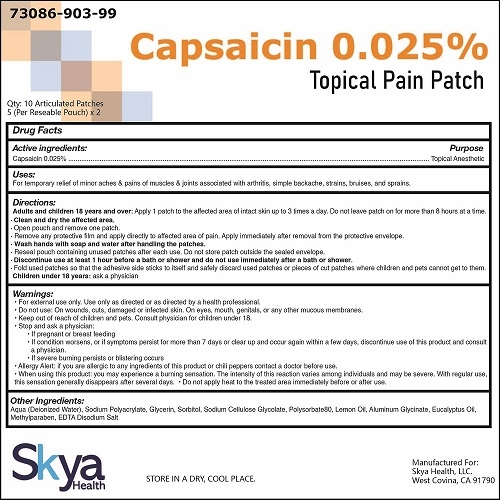
Drug Facts
Uses
Temporarily relieves minor aches and pains of muscles and joints due to:
arthritis
simple backache
strains
bruises
sprains
Warnings
For external use only. Use only as directed or as directed by a health care professional
Read all warnings and directions before use.
Discontinue use at least one hour prior to bath, shower, or swimming; do not use immediately after bath, shower, or swimming.
Do not use
On wounds, cuts, damaged or infected skin
On eyes, mouth, genitals, or any other mucous membranes
Allergy Alert: if you are allergic to capsicum or chili peppers or any inactive ingredient of this product, contact a doctor before use.
When using this product
You may experience a burning sensation. The intensity of this reaction varies among individuals and may be severe. With regular use, this sensation generally disappears after several days.
Avoid contact with the eyes, lips, nose and mucous membranes
Do not tightly wrap or bandage the treated area
Do not apply heat to the treated area immediately before or after use
Directions
Adults and children 18 years of age and older:
Apply 1 patch to the affected area of intact skin up to 3 times a day. Do not leave patch on for more than 8 hours at a time.
- Clean and dry the affected area.
- Open pouch and remove one patch.
- Remove any protective film and apply directly to affected area of pain. Apply immediately after removal from the protective envelope.
- Wash hands with soap and water after handling the patches.
- Reseal pouch containing unused patches after each use. Do not store patch outside the sealed envelope.
- Fold used patches so that the adhesive side sticks to itself and safely discard used patches or pieces of cut patches where children and pets cannot get to them.
Children under 18 years: Ask a physician
Inactive ingredients
Aqua (Deionized Water), Sodium Polyacrylate, Glycerin, Sorbitol, Sodium Cellulose Glycolate, Polysorbate 80, Lemon Oil, Aluminum Glycinate, Eucalyptus Oil, Methylparaben, EDTA Disodium Salt
PRINCIPAL DISPLAY PANEL
Capsaicin 0.025%Patch
NDC: 73086-903-99
10 Patches (5 per Resealable Pouch)
Skya Health, LLC.
| CAPSAICIN
capsaicin patch |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Skya Health, LLC (117039304) |
| Registrant - Skya Health, LLC (117039304) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Skya Health, LLC | 117039304 | repack(73086-903) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.