Cisatracurium Besalayte Injection, USP
cisatracurium besylate by
Drug Labeling and Warnings
cisatracurium besylate by is a Prescription medication manufactured, distributed, or labeled by Zydus Lifesciences Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CISATRACURIUM BESYLATE- cisatracurium besylate injection
Zydus Lifesciences Limited
----------
Cisatracurium Besalayte Injection, USP
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
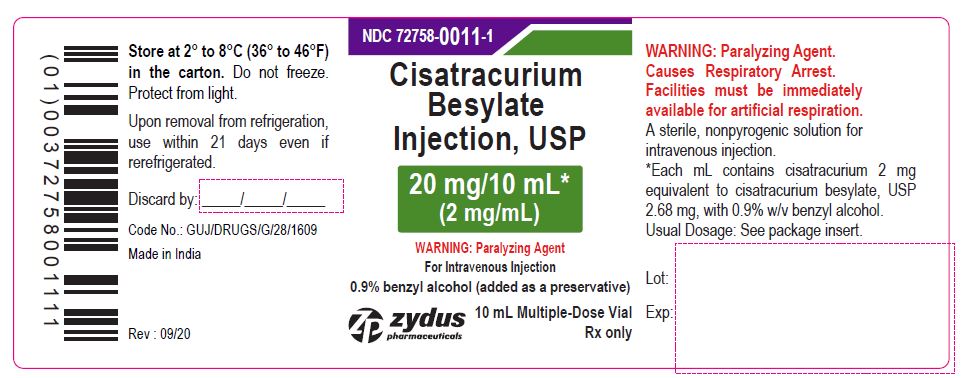
NDC: 72758-0011-1
Cisatracurium Besylate Injection, USP
20 mg/10 mL*
(2 mg/mL)
WARNING: Paralyzing Agent
For Intravenous Injection
0.9% benzyl alcohol (added as a preservative)
10 mL Multiple-Dose Vial
Rx only
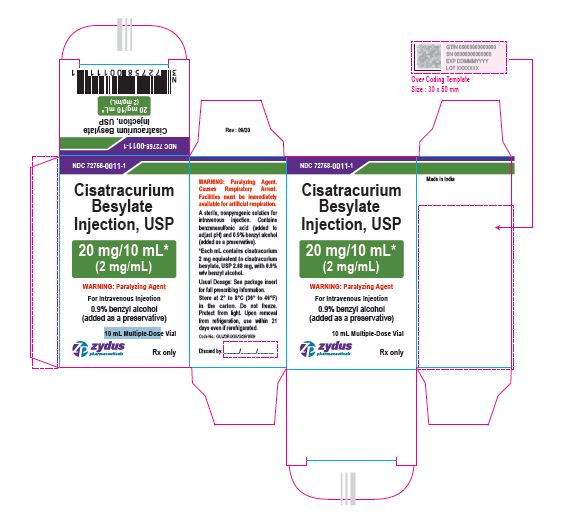
NDC: 72758-0011-1
Cisatracurium Besylate Injection, USP
20 mg/10 mL*
(2 mg/mL)
WARNING: Paralyzing Agent
For Intravenous Injection
0.9% benzyl alcohol (added as a preservative)
10 mL Multiple-Dose Vial
Rx only
NDC: 72758-0011-6
Cisatracurium Besylate Injection, USP
20 mg/10 mL*
(2 mg/mL)
WARNING: Paralyzing Agent
For Intravenous Injection
0.9% benzyl alcohol (added as a preservative)
10 x 10 mL Multiple-Dose Vial
Rx only

| CISATRACURIUM BESYLATE
cisatracurium besylate injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Zydus Lifesciences Limited (873671928) |
| Registrant - Zydus Lifesciences Limited (873671928) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Zydus Lifesciences Limited | 873671928 | MANUFACTURE(72785-0011) , ANALYSIS(72785-0011) | |