ALLOCORD- human cord blood hematopoietic progenitor cell injection, solution
ALLOCORD by
Drug Labeling and Warnings
ALLOCORD by is a Other medication manufactured, distributed, or labeled by SSM Cardinal Glennon Children's Medical Center St. Louis Cord Blood Bank. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ALLOCORD safely and effectively. See full prescribing information for ALLOCORD.
ALLOCORD (HPC, Cord Blood)
Injectable Suspension for Intravenous Use
Initial U.S. Approval: May 30, 2013WARNING: FATAL INFUSION REACTIONS, GRAFT VERSUS HOST DISEASE, ENGRAFTMENT SYNDROME, AND GRAFT FAILURE
See full prescribing information for complete boxed warning.
- Fatal infusion reactions: Monitor patients during infusion and discontinue for severe reactions. (5.1, 5.2).
- Graft-vs.-host disease (GVHD): GVHD may be fatal. Administration of immunosuppressive therapy may decrease the risk of GVHD (5.3).
- Engraftment syndrome: Engraftment syndrome may be fatal. Treat engraftment syndrome promptly with corticosteroids (5.4).
- Graft failure: Graft failure may be fatal. Monitor patients for laboratory evidence of hematopoietic recovery (5.5).
RECENT MAJOR CHANGES
Box Warning 07/2015 Contraindications (4.0) 07/2015 INDICATIONS AND USAGE
ALLOCORD,HPC, Cord Blood, is an allogeneic cord blood hematopoietic progenitor cell therapy indicated for use in unrelated donor hematopoietic progenitor cell transplantation procedures in conjunction with an appropriate preparative regimen for hematopoietic and immunologic reconstitution in patients with disorders affecting the hematopoietic system that are inherited, acquired, or result from myeloablative treatment (1).
The risk benefit assessment for an individual patient depends on the patient characteristics, including disease, stage, risk factors, and specific manifestations of the disease, on characteristics of the graft, and on other available treatments or types of hematopoietic progenitor cells (1).
DOSAGE AND ADMINISTRATION
- For intravenous use only
- Do not irradiate
- Unit selection and administration of ALLOCORD should be done under the direction of a physician experienced in hematopoietic progenitor cell transplantation (2).
- The recommended minimum dose is 2.5 x 107 nucleated cells/kg at cryopreservation (2.1).
- Do not administer ALLOCORD through the same tubing with other products except for normal saline (2.3).
DOSAGE FORMS AND STRENGTHS
Each unit contains a minimum of 5 x 108 total nucleated cells with at least 1.25 x 106 viable CD34+ cells at the time of cryopreservation. The exact pre-cryopreservation nucleated cell content of each unit is provided on the accompanying records (3).
CONTRAINDICATIONS
None (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
Mortality, from all causes, at 100 days post-transplant was 25% (5, 6.1).
The most common infusion-related adverse reactions (≥ 5%) are hypertension, vomiting, nausea, bradycardia, and fever (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact the St. Louis Cord Blood Bank at 1-888-253-CORD (1-888-253-2673) and FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
USE IN SPECIFIC POPULATIONS
Pregnancy: No animal or human data. Use only if clearly needed (8.1).
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2015
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: FATAL INFUSION REACTIONS, GRAFT VERSUS HOST DISEASE, ENGRAFTMENT SYNDROME AND GRAFT FAILURE
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosing
2.2 Preparation for Infusion
2.3 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Infusion Reactions
5.3 Graft-versus-Host Disease
5.4 Engraftment Syndrome
5.5 Graft Failure
5.6 Malignancies of Donor Origin
5.7 Transmission of Serious Infections
5.8 Transmission of Rare Genetic Diseases
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Disease
10 OVERDOSAGE
10.1 Human Overdosage Experience
10.2 Management of Overdose
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: FATAL INFUSION REACTIONS, GRAFT VERSUS HOST DISEASE, ENGRAFTMENT SYNDROME AND GRAFT FAILURE
Fatal infusion reactions: ALLOCORD administration can result in serious, including fatal, infusion reactions. Monitor patients and discontinue ALLOCORD infusion for severe reactions. [See Warnings and Precautions (5.1, 5.2)].
Graft-vs.-host disease (GVHD): GVHD is expected after administration of ALLOCORD, and may be fatal. Administration of immunosuppressive therapy may decrease the risk of GVHD [See Warnings and Precautions (5.3)].
Engraftment syndrome: Engraftment syndrome may progress to multi-organ failure and death. Treat engraftment syndrome promptly with corticosteroids [See Warnings and Precautions (5.4)].
Graft failure: Graft failure may be fatal. Monitor patients for laboratory evidence of hematopoietic recovery. Prior to choosing a specific unit of ALLOCORD, consider testing for HLA antibodies to identify patients who are alloimmunized [See Warnings and Precautions (5.5)].
-
1 INDICATIONS AND USAGE
ALLOCORD, HPC (Hematopoietic Progenitor Cell), Cord Blood, is an allogeneic cord blood hematopoietic progenitor cell therapy indicated for use in unrelated donor hematopoietic progenitor stem cell transplantation procedures in conjunction with an appropriate preparative regimen for hematopoietic and immunologic reconstitution in patients with disorders affecting the hematopoietic system that are inherited, acquired, or result from myeloablative treatment.
The risk benefit assessment for an individual patient depends on the patient characteristics, including disease, stage, risk factors, and specific manifestations of the disease, on characteristics of the graft, and on other available treatments or types of hematopoietic progenitor cells.
-
2 DOSAGE AND ADMINISTRATION
- For intravenous use only.
- Do not irradiate.
Unit selection and administration of ALLOCORD should be done under the direction of a physician experienced in hematopoietic progenitor cell transplantation.
2.1 Dosing
The recommended minimum dose is 2.5 x 107 nucleated cells/kg at cryopreservation. Multiple units may be required in order to achieve the appropriate dose.
Matching for at least 4 of 6 HLA-A antigens, HLA-B antigens, and HLA-DRB1 alleles is recommended. The HLA typing and nucleated cell content for each individual unit of ALLOCORD are documented in accompanying records.
2.2 Preparation for Infusion
ALLOCORD should be prepared by a trained healthcare professional.
- Do not irradiate ALLOCORD.
- See the appended detailed instructions for preparation of ALLOCORD for infusion.
- Once prepared for infusion, ALLOCORD may be stored at 4 to 25°C for up to 4 hours [see Instructions for Preparation for Infusion].
- The recommended limit on DMSO administration is 1 gram per kg body weight per day [see Warnings and Precautions (5.2) and Overdosage (10)].
2.3 Administration
ALLOCORD should be administered under the supervision of a qualified healthcare professional experienced in hematopoietic progenitor cell transplantation.
- Confirm the identity of the patient for the specified unit of ALLOCORD prior to administration.
- Confirm that emergency medications are available for use in the immediate area.
- Ensure the patient is hydrated adequately.
- Premedicate the patient 30 to 60 minutes before the administration of ALLOCORD. Premedication can include any or all of the following: antipyretics, histamine antagonists, and corticosteroids.
- Inspect the product for any abnormalities such as unusual particulates and for breaches of container integrity prior to administration. Prior to infusion, discuss all such product irregularities with the laboratory issuing the product for infusion.
- Administer ALLOCORD by intravenous infusion. Do not administer in the same tubing concurrently with products other than 0.9% Sodium Chloride, Injection (USP). ALLOCORD may be filtered through a 170 to 260 micron filter designed to remove clots. Do NOT use a filter designed to remove leukocytes.
- For adults, begin infusion of ALLOCORD at 100 milliliters per hour and increase the rate as tolerated. For children, begin infusion of ALLOCORD at 1 milliliter per kg per hour and increase as tolerated. Reduce the infusion rate if the fluid load is not tolerated. Discontinue the infusion in the event of an allergic reaction or if the patient develops a moderate to severe infusion reaction [See Warnings and Precautions (5.2) and Adverse Reactions (6)].
- Monitor the patient for adverse reactions during, and for at least six hours after, administration. Because ALLOCORD contains lysed red cells that may cause renal failure, careful monitoring of urine output is also recommended.
NOTE: If product is being prepared for a multi-unit infusion, infuse units independently. Should a reaction occur, appropriately manage the reaction before second unit is thawed for infusion.
-
3 DOSAGE FORMS AND STRENGTHS
Each unit of ALLOCORD contains a minimum of 5 x 108 total nucleated cells with a minimum of 1.25 x 106 viable CD34+ cells, suspended in 10% dimethyl sulfoxide (DMSO) and 1% Dextran 40, at the time of cryopreservation.
The exact pre-cryopreservation nucleated cell content is provided in accompanying records.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Allergic reactions may occur with infusion of HPC, Cord Blood, including ALLOCORD. Reactions include bronchospasm, wheezing, angioedema, pruritus and hives [see Adverse Reactions (6)]. Serious hypersensitivity reactions, including anaphylaxis, also have been reported. These reactions may be due to dimethyl sulfoxide (DMSO), Dextran 40, or a plasma component of ALLOCORD.
ALLOCORD may contain residual antibiotics if the cord blood donor was exposed to antibiotics in utero. Patients with a history of allergic reactions to antibiotics should be monitored for allergic reactions following ALLOCORD administration.
5.2 Infusion Reactions
Infusion reactions are expected to occur and include nausea, vomiting, fever, rigors or chills, flushing, dyspnea, hypoxemia, chest tightness, hypertension, tachycardia, bradycardia, dysgeusia, hematuria, and mild headache. Premedication with antipyretics, histamine antagonists, and corticosteroids may reduce the incidence and intensity of infusion reactions.
Severe reactions, including respiratory distress, severe bronchospasm, severe bradycardia with heart block or other arrhythmias, cardiac arrest, hypotension, hemolysis, elevated liver enzymes, renal compromise, encephalopathy, loss of consciousness, and seizure also may occur. Many of these reactions are related to the amount of DMSO administered. Minimizing the amount of DMSO administered may reduce the risk of such reactions, although idiosyncratic responses may occur even at DMSO doses thought to be tolerated. The actual amount of DMSO depends on the method of preparation of the product for infusion. Limiting the amount of DMSO infused to no more than 1 gram per kilogram per day is recommended [see Overdosage (10)].
Infusion reactions may begin within minutes of the start of infusion of ALLOCORD, although symptoms may continue to intensify and not peak for several hours after completion of the infusion. Monitor the patient closely during this period. If a reaction occurs, discontinue the infusion and institute supportive care as needed.
If infusing more than one unit of HPC, Cord Blood, on the same day, do not administer subsequent units until all signs and symptoms of infusion reactions from the prior unit have resolved.
5.3 Graft-versus-Host Disease
Acute and chronic graft-versus-host disease (GVHD) may occur in patients who have received ALLOCORD. Classic acute GVHD is manifested as fever, rash, elevated bilirubin and liver enzymes, and diarrhea. Patients transplanted with ALLOCORD also should receive immunosuppressive drugs to decrease the risk of GVHD [See Adverse Reactions (6.1)].
5.4 Engraftment Syndrome
Engraftment syndrome is manifested as unexplained fever and rash in the peri-engraftment period. Patients with engraftment syndrome also may have unexplained weight gain, hypoxemia, and pulmonary infiltrates in the absence of fluid overload or cardiac disease. If untreated, engraftment syndrome may progress to multi-organ failure and death. Begin treatment with corticosteroids once engraftment syndrome is recognized in order to ameliorate the symptoms [See Adverse Reactions (6.1)].
5.5 Graft Failure
Primary graft failure, which may be fatal, is defined as failure to achieve an absolute neutrophil count greater than 500 per microliter blood by Day 42 after transplantation. Immunologic rejection is the primary cause of graft failure. Patients should be monitored for laboratory evidence of hematopoietic recovery. Consider testing for HLA antibodies in order to identify patients who are alloimmunized prior to transplantation and to assist with choosing a unit with a suitable HLA type for the individual patient [See Adverse Reactions (6.1)].
5.6 Malignancies of Donor Origin
Patients who have undergone HPC, Cord Blood, transplantation may develop post-transplant lymphoproliferative disorder (PTLD), manifested as a lymphoma-like disease favoring non-nodal sites. PTLD is usually fatal if not treated.
The incidence of PTLD appears to be higher in patients who have received antithymocyte globulin. The etiology is thought to be donor lymphoid cells transformed by Epstein-Barr virus (EBV). Serial monitoring of blood for EBV DNA may be warranted in high-risk groups.
Leukemia of donor origin also has been reported in HPC, Cord Blood recipients. The natural history is presumed to be the same as that for de novo leukemia.
5.7 Transmission of Serious Infections
Transmission of infectious disease may occur because ALLOCORD is derived from human blood. Disease may be caused by known or unknown infectious agents. Donors are screened for increased risk of infection with human immunodeficiency virus (HIV), human T-cell lymphotropic virus (HTLV), hepatitis B virus (HBV), hepatitis C virus (HCV), T. pallidum, T. cruzi, West Nile Virus (WNV), transmissible spongiform encephalopathy (TSE) agents, and vaccinia. Donors are also screened for clinical evidence of sepsis, and communicable disease risks associated with xenotransplantation. Maternal blood samples are tested for HIV types 1 and 2, HTLV types I and II, HBV, HCV, T. pallidum, WNV, and T. cruzi. ALLOCORD is tested for sterility. These measures do not totally eliminate the risk of transmitting these or other transmissible infectious diseases and disease agents. Report the occurrence of a suspected transmitted infection to the St. Louis Cord Blood Bank of the SMM Cardinal Glennon Children's Medical Center at 1-888-253-CORD (1-888-253-2673).
Testing is also performed for evidence of donor infection due to cytomegalovirus (CMV). The result may be found in accompanying records.
5.8 Transmission of Rare Genetic Diseases
ALLOCORD may transmit rare genetic diseases involving the hematopoietic system for which donor screening and/or testing has not been performed [see Adverse Reactions (6.1)]. Cord blood donors have been screened by family history to exclude inherited disorders of the blood and marrow. ALLOCORD has been tested to exclude donors with sickle cell anemia, and anemias due to abnormalities in hemoglobins C, D, and E. Because of the age of the donor at the time ALLOCORD collection takes place, the ability to exclude rare genetic diseases is severely limited.
-
6 ADVERSE REACTIONS
Day-100 mortality from all causes was 25%.
The most common infusion-related adverse reactions (≥ 5%) are hypertension, vomiting, nausea, bradycardia, and fever.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety assessment of ALLOCORD is based primarily on review of the data submitted to the FDA dockets from various sources, the dataset for the COBLT Study, and published literature.
Infusion Reactions
The data described in Table 1 reflect exposure to 442 infusions of HPC, Cord Blood, (from multiple cord blood banks) in patients treated using a total nucleated cell dose ≥ 2.5 x 107/kg on a single-arm prospectivetrial or expanded access use (COBLT Study). The population was 59% male and the median age was 5 years (range 0.05-68 years), and included patients treated for hematologic malignancies, inherited metabolic disorders, primary immunodeficiencies, and bone marrow failure. Preparative regimens and graft-vs.-host disease prophylaxis were not standardized. The most common infusion reactions were hypertension, vomiting, nausea, and sinus bradycardia. Hypertension and any grades 3-4 infusion-related reactions occurred more frequently in patients receiving HPC, Cord Blood, in volumes greater than 150 milliliters and in pediatric patients. The rate of serious adverse cardiopulmonary reactions was 0.8%.
Table 1: Incidence of Infusion-Related Adverse Reactions Occurring in ≥1% of Infusions (COBLT Study) Any grade Grade 3-4 Any reaction 65.4% 27.6% Hypertension 48.0% 21.3% Vomiting 14.5% 0.2% Nausea 12.7% 5.7% Sinus bradycardia 10.4% 0 Fever 5.2% 0.2% Sinus tachycardia 4.5% 0.2% Allergy 3.4% 0.2% Hypotension 2.5% 0 Hemoglobinuria 2.1% 0 Hypoxia 2.0% 2.0% Information on infusion reactions was available from voluntary reports for 737 patients who received ALLOCORD. Preparative regimens and graft-vs.-host disease prophylaxis were not standardized. The reactions were not graded. An infusion reaction occurred in 13% of patients. The most common infusion reactions, occurring in ≥ 1% of patients, were hypertension (54%), vomiting (12%), dyspnea (9%), bradycardia (6%), nausea (4%), chest pain (2%), hemoglobinuria (2%), fever (2%) and hives (2%).
Other Adverse Reactions
For other adverse reactions, the raw clinical data from the dockets were pooled for 1299 (120 adult and 1179 pediatric) patients transplanted with HPC, Cord Blood, (from multiple cord blood banks) with total nucleated cell dose ≥ 2.5 x 107/kg. Of these, 66% (n=862) underwent transplantation as treatment for hematologic malignancy. The preparative regimens and graft-vs.-host disease prophylaxis varied. The median total nucleated cell dose was 6.4 x 107/kg (range, 2.5-73.8 x 107/kg). For these patients, Day-100 mortality from all causes was 25%. Primary graft failure occurred in 16%; 42% developed grades 2-4 acute graft-vs.-host disease; and 19% developed grades 3-4 acute graft-vs.-host disease.
Data from published literature and from observational registries, institutional databases, and cord blood bank reviews reported to the dockets for HPC, Cord Blood, (from multiple cord blood banks) revealed nine cases of donor cell leukemia, one case of transmission of infection, and one report of transplantation from a donor with an inheritable genetic disorder. The data are not sufficient to support reliable estimates of the incidences of these events.
In the COBLT Study, 15% of the patients developed engraftment syndrome.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C. Animal reproduction studies have not been conducted with ALLOCORD. It is also not known whether ALLOCORD can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. There are no adequate and well-controlled studies in pregnant women. ALLOCORD should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
8.4 Pediatric Use
HPC, Cord Blood, has been used in pediatric patients with disorders affecting the hematopoietic system that are inherited, acquired, or resulted from myeloablative treatment [See Dosage and Administration (2), Adverse Reactions (6), and Clinical Studies (14)].
8.5 Geriatric Use
Clinical studies of HPC, Cord Blood, (from multiple cord blood banks) did not include sufficient numbers of subjects aged 65 years and over to determine whether they respond differently than younger subjects. In general, administration of ALLOCORD to patients over age 65 years should be cautious, reflecting their greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
10 OVERDOSAGE
10.1 Human Overdosage Experience
There has been no experience with overdosage of HPC, Cord Blood, in human clinical trials. Single doses of ALLOCORD up to 67.0 x 107 TNC/kg have been administered. HPC, Cord Blood, prepared for infusion may contain dimethyl sulfoxide (DMSO). The maximum tolerated dose of DMSO has not been established, but it is customary not to exceed a DMSO dose of 1 gm/kg/day when given intravenously. Several cases of altered mental status and coma have been reported with higher doses of DMSO.
-
11 DESCRIPTION
ALLOCORD consists of hematopoietic progenitor cells, monocytes, lymphocytes, and granulocytes from human cord blood for intravenous infusion. Blood recovered from umbilical cord and placenta is volume reduced and partially depleted of red blood cells and plasma.
The active ingredient is hematopoietic progenitor cells which express the cell surface marker CD34. The potency of cord blood is determined by measuring the numbers of total nucleated cells (TNC) and CD34+ cells, and cell viability. Each unit of ALLOCORD contains a minimum of 5 x 108 total nucleated cells with at least 1.25 x 106 viable CD34+ cells at the time of cryopreservation. The cellular composition of ALLOCORD depends on the composition of cells in the blood recovered from the umbilical cord and placenta of the donor. The actual nucleated cell count, the CD34+ cell count, the ABO group, and the HLA typing are listed in accompanying records sent with each individual unit.
ALLOCORD has the following inactive ingredients: PrepaCyte-CB separation solution, citrate-phosphate-dextrose, dimethyl sulfoxide (DMSO) and Dextran 40. When prepared for infusion according to instructions, the infusate contains the following inactive ingredients: PrepaCyte-CB separation solution, citrate-phosphate-dextrose, Dextran 40, human serum albumin, and residual DMSO.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Hematopoietic stem/progenitor cells from HPC, Cord Blood, migrate to the bone marrow where they divide and mature. The mature cells are released into the bloodstream, where some circulate and others migrate to tissue sites, partially or fully restoring blood counts and function, including immune function, of blood-borne cells of marrow origin [See Clinical Studies (14)].
In patients with enzymatic abnormalities due to certain severe types of storage disorders, mature leukocytes resulting from HPC, Cord Blood, transplantation may synthesize enzymes that may be able to circulate and improve cellular functions of some native tissues. However, the precise mechanism of action is unknown.
-
14 CLINICAL STUDIES
The effectiveness of HPC, Cord Blood, as defined by hematopoietic reconstitution, was demonstrated in one single-arm prospective study (COBLT Study), and in retrospective reviews of data from an observational database for ALLOCORD and data in the dockets and public information. Of the 1299 patients in the dockets and public data, 66% (n=862) underwent transplantation as treatment for hematologic malignancy. Results for patients who received a total nucleated cell dose ≥2.5 x 107/kg are shown in Table 2. Neutrophil recovery is defined as the time from transplantation to an absolute neutrophil count more than 500 per microliter. Platelet recovery is the time to a platelet count more than 20,000 per microliter. Erythrocyte recovery is the time to a reticulocyte count greater than 30,000 per microliter. The total nucleated cell dose and degree of HLA match were inversely associated with the time to neutrophil recovery in the docket data.
Table 2: Hematopoietic Recovery for Patients Transplanted with HPC, Cord Blood, Total Nucleated Cell (TNC) Dose ≥ 2.5 x 107/kg * HPC, Cord Blood (from multiple cord blood banks)
** The analysis of hematopoietic recovery is based on a different number of patients, ranging from 335 to 442, for each variable because the amount of data missing is different for each variable.
Data Source COBLT
Study*Docket* and
Public Data*ALLOCORD Design Single-arm
prospectiveRetrospective Retrospective Number of patients 324 1299 1086 Median age (years)
(range)4.6
(0.07 – 52.2)7.0
(<1 – 65.7)6.6
(0.05 – 70)Gender 59% male
41% female57% male
43% female54% male
43% female
3% unknownMedian TNC Dose (x 107/kg)
(range)6.7
(2.6 – 38.8)6.4
(2.5 – 73.8)6.4
(2.5 – 67.0)Neutrophil Recovery at Day 42 (95% CI) 76%
(71% – 81%)77%
(75% – 79%)88%**
(85% – 91%)Platelet Recovery at Day 100 of 20,000/microliter (95% CI) 57%
(51% – 63%)
-87%**
(83% – 91%)Platelet Recovery at Day 100 of 50,000/microliter (95% CI) 46%
(39% – 51%)45%
(42% – 48%)79%**
(73% – 83%)Erythrocyte Recovery at Day 100
(95% CI)65%
(58% – 71%)- - Median time to Neutrophil Recovery 27 days 25 days 21 days** Median time to Platelet Recovery of 20,000/microliter 90 days - 48 days** Median time to Platelet Recovery of 50,000/microliter 113 days 122 days 56 days** Median time to Erythrocyte Recovery 64 days - - -
16 HOW SUPPLIED/STORAGE AND HANDLING
ALLOCORD is supplied as a cryopreserved cell suspension in a sealed bag containing a minimum of 5 x 108 total nucleated cells with a minimum of 1.25 x 106 viable CD34+ cells in a volume of 35 milliliters (ISBT 128 Product Code S1393, ISBT 128 Facility Identifier Number W1205). The exact pre-cryopreservation nucleated cell content is provided in accompanying records.
Store ALLOCORD at or below -150 °C until ready for thawing and preparation.
-
17 PATIENT COUNSELING INFORMATION
Discuss the following with patients receiving ALLOCORD:
- Report immediately any signs and symptoms of acute infusion reactions, such as fever, chills, fatigue, breathing problems, dizziness, nausea, vomiting, headache, or muscle aches.
- Report immediately any signs or symptoms suggestive of graft-vs.-host disease, including rash, diarrhea, or yellowing of the eyes.
-
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR PREPARATION FOR INFUSION
1 EQUIPMENT, REAGENTS, AND SUPPLIES
EQUIPMENT:
Biologic safety cabinet
Waterbath, 37°C
Heat sealer
Scale
Automated cell counter
Flow cytometer
Microscope
REAGENTS:
25% Albumin (Human), USP
Dextran 40 in Sodium Chloride Injection, USP or Dextran 40 in Dextrose Injection, USP
SUPPLIES:
Sterile sealable zip lock bag
Syringes - 1 mL, 3 mL, 5 mL, 30 mL, 60 mL
18 gauge safety needles
Blunt plastic cannulas
Sterile syringe caps (dual end: male/female)
Alcohol wipes
Plasma transfer sets (2 inch tubing, female luer adapter) – included with product shipment
Transfer packs – 150 mL, 300 mL
Blood culture vials
Blood culture device
Hemostat
2 mL cryovial
2 RECEIPT INSTRUCTIONS
ALLOCORD is shipped frozen in a steel canister that is contained in an insulating foam sleeve. ALLOCORD must be stored at or below -150 °C, either inside the container used for shipping (dry-shipper) or in a liquid nitrogen (LN2)-cooled storage device at the Transplant Center (recommended).
Upon receiving the shipment, perform the following steps:
- Confirm receipt of the shipment and the identity of the expected unit.
- Inspect the shipper for tampering or damage prior to opening.
- Weigh the shipper and document the weight on the Unit Receipt Form.
- Note the temperature displayed on the data logger and document the temperature on the Unit Receipt Form.
- Using cryoprotective gloves, remove the product from the canister and place in a reservoir with LN2 or in the vapor phase of aLN2freezer.
- Carefully open the cassette. Inspect the integrity of the unit(s) received and document its conditions on the Unit Receipt Form.
- Confirm the identity of the cord blood unit. Include this check on the Unit Receipt Form.
- Store the product in an LN2 storage vessel that maintains a temperature below -150°C.
- Reserve the samples that accompany the unit as a DNA source for confirmatory testing or post engraftment studies:
- a segment remains on the unit; reserve it prior to thawing the unit
- an aliquot containing the material remaining from the red blood cell/plasma reduction
- an aliquot containing unmanipulated cord blood collected in Citrate Phosphate Dextrose (CPD)
- a spot card containing unmanipulated cord blood collected in CPD (in envelope)
NOTE: Aside from the segment, ancillary samples are not intended to represent the cell count or potency of the cryopreserved product.
- Replace data logger temperature probe wire inside the inner dry shipper container if necessary, and reassemble the shipper for return.
- Fax the completed Unit Receipt Form to St. Louis Cord Blood Bank of the SSM Cardinal Glennon Children's Medical Center at 314-268-4186.
NOTE: If there is any error or ambiguity with regard to the product documentation, close the canister and keep the product at LN2 temperature. Immediately advise the staff of the St. Louis Cord Blood Bank and the transplant physician. Do not proceed until the problem is resolved. If your LN2 storage tanks have no space to store the product in its canister and insulated sleeve, add LN2 to the St. Louis Cord Blood Bank dry-shipper to keep the product frozen until a completely satisfactory determination is made.
3 PREPARATION
- Coordination with the clinical team
- Confirm the infusion time in advance, adjusting the start time for thaw so the unit is available for infusion when the recipient is ready.
- Consult with the clinicians about final product volume based on the recipient's weight and possible fluid restrictions.
- General Information
- Use aseptic technique in a biological safety cabinet for all processing steps, including all open-container processing and all spiking of container ports.
- Use only sterile materials when processing the cellular product.
- Record the manufacturer information, lot number and expiration date (if applicable) of all reagents and disposables.
- Prepare the water bath and verify that the temperature is 37°C.
- Prepare the reconstitution solution
- Combine 250 mL Dextran 40 and 50 mL 25% albumin in a 300 mL transfer pack. Clamp the tubing with a hemostat.
- Using appropriate sized syringes, withdraw the following and cap the syringes with blunt plastic cannula:
- 50 mL of reconstitution solution. If the total frozen volume (product + DMSO volume) exceeds the standard 50mL reconstitution solution, use a volume of reconstitution solution equal to the total frozen volume so the dilution ratio is at least 1:1
- 30 mL of reconstitution solution to be used as a container rinse for microbial culturing
- Obtain product
- Prepare a portable canister with LN2 using appropriate personal protective equipment (gloves, gowns, face shield).
- Product verification requires two members of the laboratory staff. With product and recipient files at hand, locate and remove the product from its location in the freezer, but maintaining in vapor phase. Expeditiously verify product identity, labeling, accuracy of information, and container integrity.
- Remove any segment attached to the unit, place into a 2 mL cryovial and store in either vapor or liquid phase of nitrogen (<-150°C).
- Immediately transfer the product from LN2 storage tank into the portable canister containing LN2.
4 PROCEDURE
Reconstitution or simple dilution of ALLOCORD with dextran/human serum albumin (HSA) solution, using the Thawing and Diluting procedures described below, is recommended. The Alternate Procedure – Washing may be considered if the infusion volume and/or DMSO dose are contraindicated (>1 mL/kg).
NOTE: Minimize the time from initiation of thaw to completion of infusion.
THAWING:
- Verify the identity of the product being thawed.
- Remove the ALLOCORD unit from the cassette. Examine the cryobag for breaks or cracks.
- Carefully place the unit inside a sterile sealable zip-lock bag, and submerge in the 37°C water bath, keeping port(s) dry and above water.
- Document the thaw start time.
- To accelerate thawing, gently knead contents of bag.
NOTE: Inspect for leaks! If container integrity is observed to be compromised, position the cryobag and/or clamp with hemostats to prevent further escape of blood.
- When the contents of the cryobag become slushy, remove the bag from the 37ºC water bath.
- Note the thaw stop time. Product expiration time is four hours from this step.
- Gently wipe the outside surface of the cryobag with alcohol, and place the cryobag into the biologic safety cabinet.
DILUTING:
- Insert a plasma transfer set into the cryobag.
- Attach the syringe containing the 50mL reconstitution solution to the transfer set on the cryobag.
- Slowly introduce approximately half the volume of the reconstitution solution to the thawed product while mixing the fluids in the bag.
- Insert the spike of a correctly labeled appropriate volume capacity transfer pack into the second access port of the cryobag.
- Weigh the empty transfer pack to determine the tare weight of the bag.
- Drain the contents from the cryobag into transfer pack.
- Clamp the tubing between the bags with a hemostat.
- Add the remaining reconstitution solution to the cryobag.
- Mix well to rinse the cells from the bag and drain into transfer pack.
- Clamp the tubing between the bags.
- Weigh the transfer pack subtracting the tare weight to get product volume.
- Insert a transfer set into the product transfer pack.
- Aseptically attach a 3 mL syringe and aspirate a 1 mL aliquot for quality control testing.
- Deliver the 1mL testing aliquot into a labeled aliquot tube.
- Subtract the 1mL (testing aliquot) from product volume to determine infusion volume. Record the infusion volume which will be used for calculating cell numbers.
- Heat seal the tubing between the cryobag and the transfer pack.
- Cut tubing at the seals and separate the bags.
NOTE: At this point, it is approximately 30 minutes from infusion. Notify the clinical transplant team to pre-medicate the patient as ordered.
- Aseptically introduce the 30 mL of reconstitution solution from the syringe prepared in step 3.c.ii.2) into the (now empty) original product cryobag.
- Immediately transport the product to the clinical transplant site per the facility's SOP.
ALTERNATIVE PROCEDURE - WASHING:
Perform steps a. through r. of the Thawing Procedure and Diluting Procedure as outlined above, then complete the following:
- Place the transfer pack into a sterile overwrap bag ready for centrifugation.
- Support bag in centrifuge bucket insert to prevent the formation of creases during centrifugation.
- Balance carriers and centrifuge at 650 x g (1500 rpm) for 20 minutes at 10°C (no brake).
- Carefully remove the transfer pack from the centrifuge into the biologic safety cabinet, placing the transfer pack into a plasma expresser.
- Using the original cryobag to collect the waste volume, express 75% of the volume of reconstitution solution originally added to the thawed product pre-centrifugation. Avoid accidental passage of cells with the supernatant.
- Allow the cells to rest for five minutes. Resuspend the sedimented cell pellet by gentle agitation.
- Obtain quality control samples as described in steps s. through aa. above.
5 QUALITY CONTROL:
Perform quality control assays per transplant center policies and procedures using the aliquot of thawed product obtained in step u above. Recommended assays include:
- Nucleated Cell count
- Viability test
- Viable CD34+ cell count
- Colony Forming Unit
- Microbial cultures (aerobic, anaerobic and fungal)
CALCULATIONS:
Infusion TNC [x109] = (WBC/mL + NRBC/mL [x106]) x infusion volume (mL)
TNC dose [x107/kg] = Infusion TNC [x109]
Recipient wt (kg)Post-thaw TNC recovery [%] = TNC of thawed product [x109] x 100
TNC of original frozen product [x109]Total CD34+ cells [x106] = CD34+ cells/mL x dilution factor x 1000 mL x product volume (mL)
CD34+ cell dose [x105/kg] = Absolute CD34+ cell cells (x106) ÷ Recipient weight (kg)
Product RBC volume [mL] = product hematocrit x product volume (mL)
RBC dose [mL/kg] = Product RBC volume [mL] ÷ Recipient weight (kg)
Product CFU count [x105] = Colonies scored per 105 NC x product TNC [x109]
105CFU dose [x104/kg] = Product CFU count [x105] ÷ Recipient weight (kg)
6 CONTACT INFORMATION
SSM Cardinal Glennon Children's Medical Center
St. Louis Cord Blood Bank (SLCBB)
3662 Park Avenue
St. Louis, MO 63110SLCBB Hours: Monday-Friday, 7:00am – 5:00pm, Central Time
SLCBB Phone Number: 314-268-2787 or 888-453-CORD (888-453-2673)
SLCBB Fax Number: 314-268-4186After Hours Numbers:
Director: 314-486-2488
Distribution: 314-277-1638DISTRIBUTED BY:
SSM Cardinal Glennon Children's Medical Center
dba St. Louis Cord Blood Bank
1465 South Grand Blvd
St. Louis, MO 63104
US License 1873 -
PRINCIPAL DISPLAY PANEL
Principal Display Panel - Canister Label
6200
A
Rh POSITIVEFor Use By Intended Recipient Only
Properly Identified Intended
Recipient and ProductExpiration Date/Time
26 Feb 2017 10:15 CST
(26 Feb 2014 16:15 UTC)See package insert for full prescribing
information and instructions for
preparationProcessed By:
SSM Cardinal Glennon Children's Medical Center
St. Louis Cord Blood Bank3662 Park Avenue
St. Louis, MO 63110US Lic# 1873
"W1205 14 000014"
Collection Date/Time
25 Feb 2014 15:45 CST
(25 Feb 2014 21:45 UTC)Do Not Irradiate
Do Not Use Leukoreduction Filter
S1393V00
Cryopreserved
HPC, Cord Blood
Approx. 29 mL including
10% DMSO, 1% Dextran 40;
approx 42% Prepacyte®-CB and 10% CPDStore at ≤ -150C
Rx Only
For Intravenous administration
-
PRINCIPAL DISPLAY PANEL
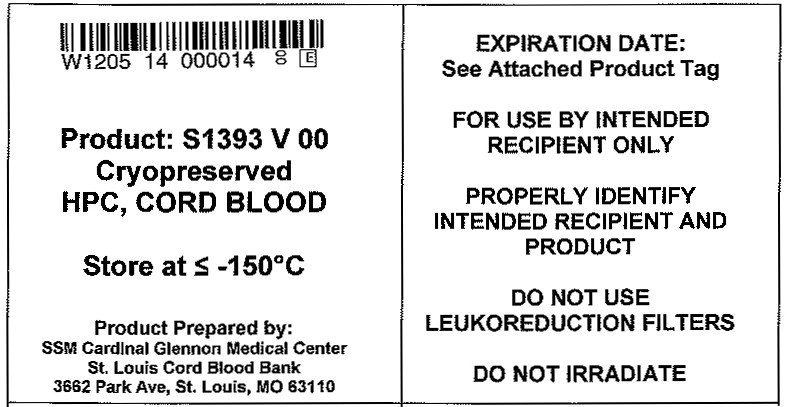
Principal Display Panel - Bag Label
"W1205 14 000014"
Product: S1393 V 00
Cryopreserved
HPC, CORD BLOODStore at ≤ -150°C
Product Prepared by:
SSM Cardinal Glennon Children's Medical Center
St. Louis Cord Blood Bank3662 Park Avenue, St. Louis, MO 63110
EXPIRATION DATE:
See Attached Product Tag
FOR USE BY INTENDED
RECIPIENT ONLYPROPERLY IDENTIFY
INTENDED RECIPIENT AND
PRODUCTDO NOT USE
LEUKOREDUCTION FILTERSDO NOT IRRADIATE
-
INGREDIENTS AND APPEARANCE
ALLOCORD
human cord blood hematopoietic progenitor cell injection, solutionProduct Information Product Type LICENSED MINIMALLY MANIPULATED CELLS Item Code (Source) ISBT:W1205-S1393 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Human Cord Blood Hematopoietic Progenitor Cell (UNII: XU53VK93MC) (Human Cord Blood Hematopoietic Progenitor Cell - UNII:XU53VK93MC) Human Cord Blood Hematopoietic Progenitor Cell 500000000 in 35 mL Inactive Ingredients Ingredient Name Strength Dimethyl Sulfoxide (UNII: YOW8V9698H) Dextran 40 (UNII: K3R6ZDH4DU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 ISBT:W1205-S1393-02 1 in 1 CANISTER 1 ISBT:W1205-S1393-01 35 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125413 05/31/2013 Labeler - SSM Cardinal Glennon Children's Medical Center St. Louis Cord Blood Bank (075904839) Establishment Name Address ID/FEI Business Operations SSM Cardinal Glennon Children's Medical Center St. Louis Cord Blood Bank 075904839 MANUFACTURE(W1205-S1393) , LABEL(W1205-S1393) , PACK(W1205-S1393)
Trademark Results [ALLOCORD]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ALLOCORD 87159433 5181158 Live/Registered |
SSM Health Care Corporation 2016-09-02 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.