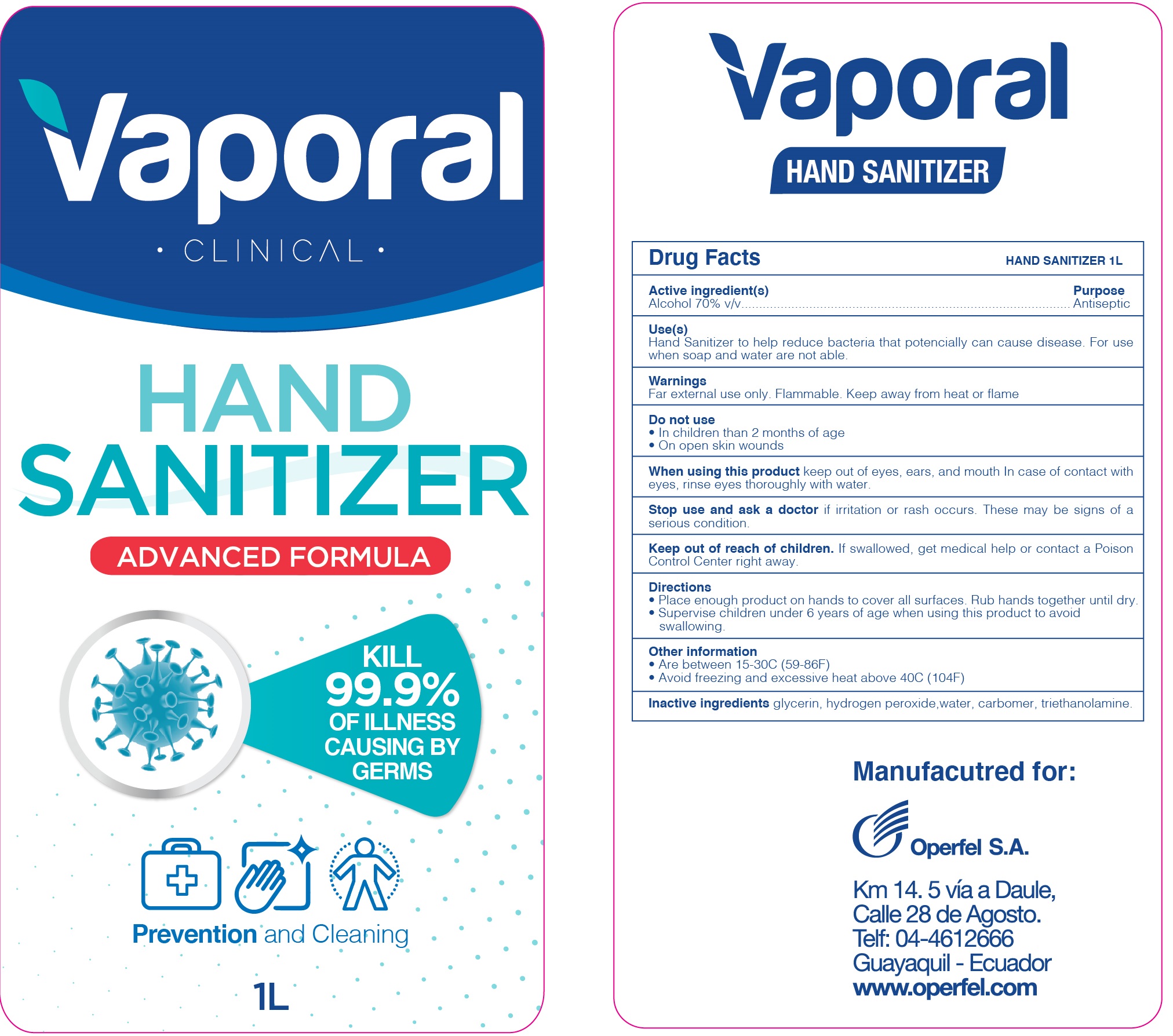
Vaporal Hand Sanitizer by Operfel S.A. Vaporal Hand Sanitizer
Vaporal Hand Sanitizer by
Drug Labeling and Warnings
Vaporal Hand Sanitizer by is a Otc medication manufactured, distributed, or labeled by Operfel S.A.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
VAPORAL HAND SANITIZER- alcohol gel
Operfel S.A.
----------
Vaporal Hand Sanitizer
Use(s)
Hand Sanitizer to help reduce bacteria that potentially can cause disease. For use when soap and water are not able.
Warnings
For external use only. Flammable. Keep away from heat or flame
| VAPORAL HAND SANITIZER
alcohol gel |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Operfel S.A. (935284554) |
Revised: 1/2024
<
Document Id: 0e1197e8-7b5d-14aa-e063-6294a90a9654
Set id: ac893343-e9cc-4f1e-e053-2995a90a41a1
Version: 2
Effective Time: 20240103
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.