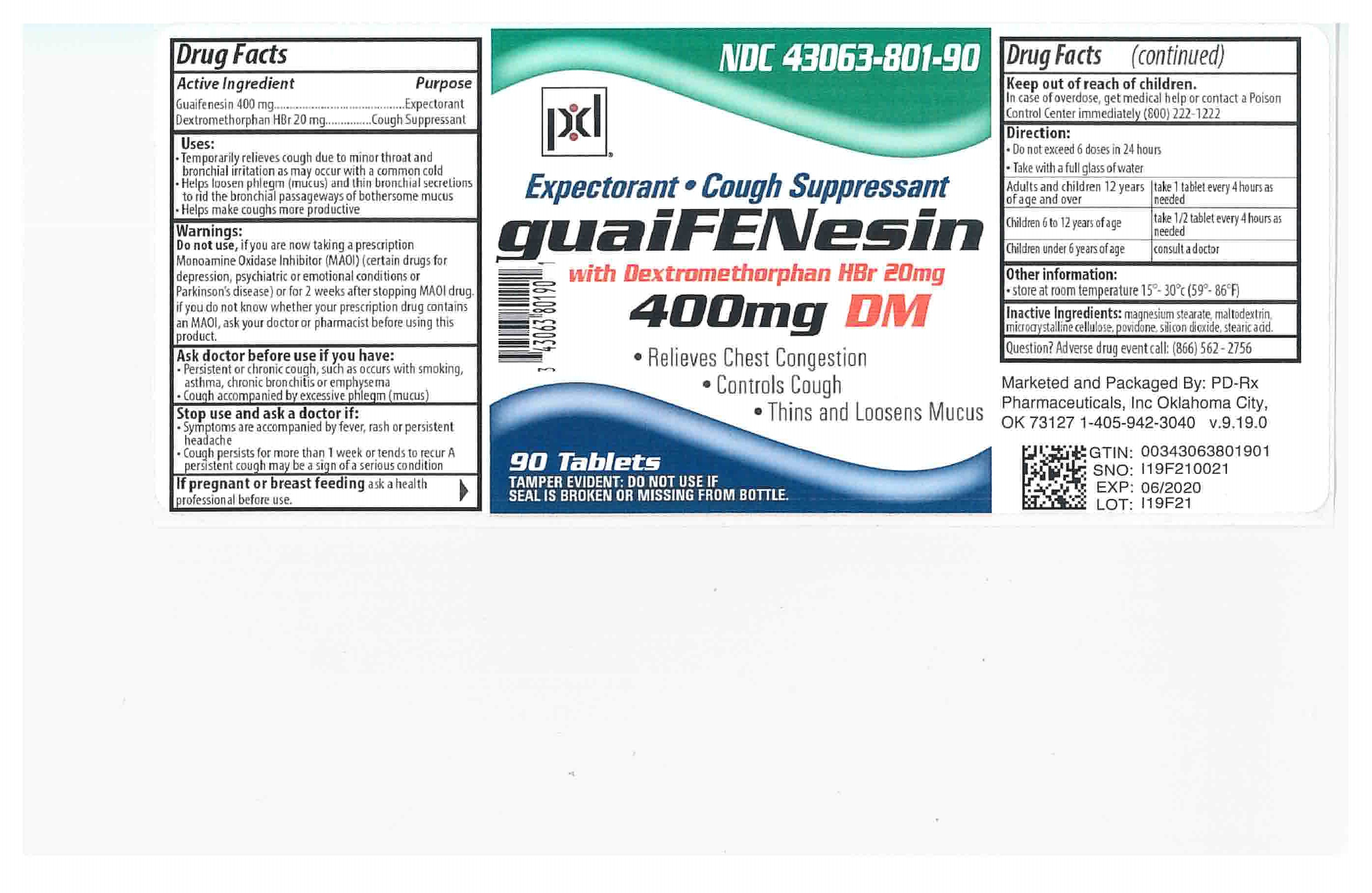
GUAIFENESIN DM- guaifenesin and dextromethorphan hydrobromide tablet
Guaifenesin DM by
Drug Labeling and Warnings
Guaifenesin DM by is a Otc medication manufactured, distributed, or labeled by PD-Rx Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredient Section
- Keep Out Of Reach of Children
-
Indications & Usage
Temporarily relieves cough due to minor throat and bronchial irritation as may occur with the common cold
Helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus
Helps make coughs more productive.
These highlights do not include all the information needed to use Guaifenesin DM, See full prescribing information for Guaifenesin DM Initial U.S. Approval 04/29/2004
-
Warnings
WARNINGS:
Do not use, if you are now taking a prescription Monoamine Oxidase Inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- Ask doctor before use.
- Stop Use Section
- Pregnancy or breast feeding
- Questions?
- Storage
- Inactive Ingredient
- Purpose
-
DOSAGE & ADMINISTRATION
Directions: Adults and children 12 years of age and older, take 1 tablet every 4 hours as needed with a full glass of water while symptoms persist.
Do not exceed 6 doses in 24 hours.
Children 6 to 12 years of age take 1/2 tablet every 4 hours as needed with a full glass of water.
Children under 6 years of age consult a doctor.
These highlights do not include all the information needed to use Guaifenesin DM
See full prescribing information for Guaifenesin DM
Initial U.S. Approval 04/29/04
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUAIFENESIN DM
guaifenesin and dextromethorphan hydrobromide tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 43063-801 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 400 mg DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 20 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POVIDONE (UNII: FZ989GH94E) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color white Score no score Shape CAPSULE Size 17mm Flavor Imprint Code PH;073 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 43063-801-90 90 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/14/2017 11/30/2027 2 NDC: 43063-801-60 60 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/14/2017 11/30/2027 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 11/14/2017 11/30/2027 Labeler - PD-Rx Pharmaceuticals, Inc. (156893695) Registrant - PD-Rx Pharmaceuticals, Inc. (156893695) Establishment Name Address ID/FEI Business Operations PD-Rx Pharmaceuticals, Inc. 156893695 repack(43063-801)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.