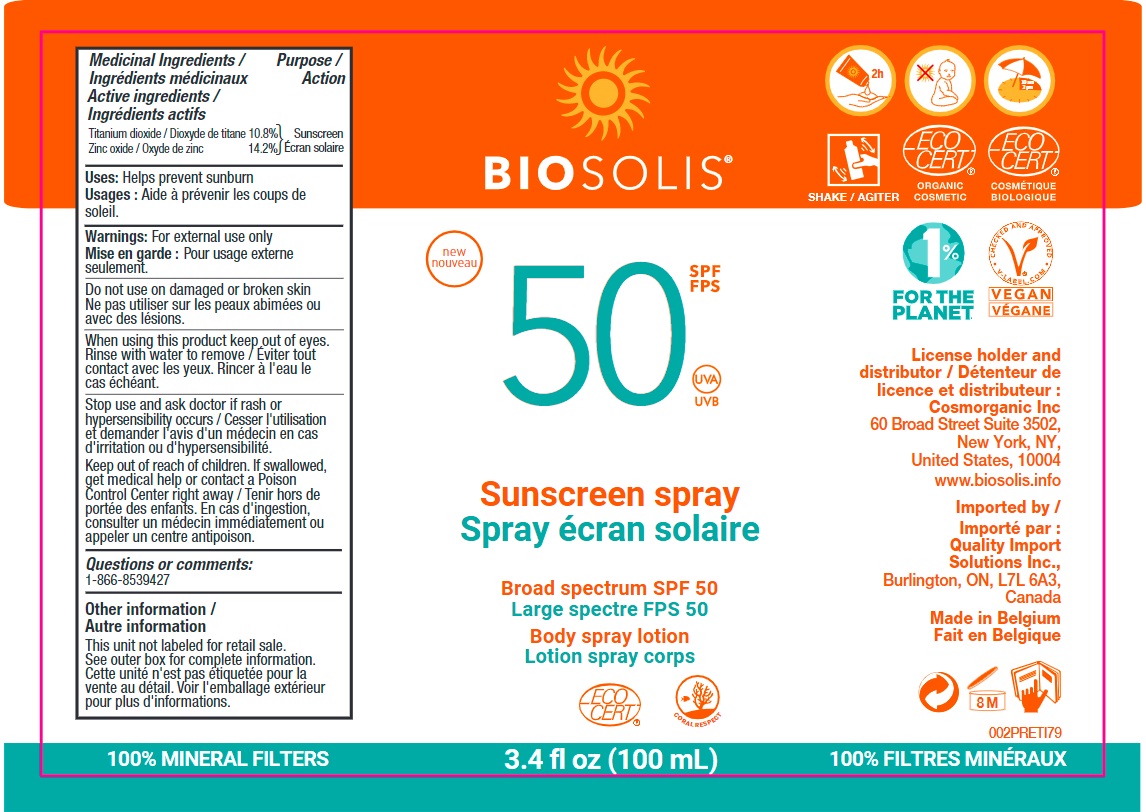
BIOSOLIS sunscreen spray broadspectrum SPF50
BIOSOLIS sunscreen broadspectrum SPF50 by
Drug Labeling and Warnings
BIOSOLIS sunscreen broadspectrum SPF50 by is a Otc medication manufactured, distributed, or labeled by Pro Vera SA. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
BIOSOLIS SUNSCREEN BROADSPECTRUM SPF50- titanium dioxide, zinc oxide cream
Pro Vera SA
----------
BIOSOLIS sunscreen spray broadspectrum SPF50
Uses
- helps prevent sunburn.
- if used as directed with other sun protection measures(see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Directions
- apply liberally 15 minutes before sun exposure
- Sun protection measures: Spending time in the sun increases your risk of skin cancer and early skin aging.To decrease this risk, regularly use a sunscreen with aBroad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 3 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- reapply
- after swimming or sweating
- immediately after towel drying
- at least every 2 hours
- children under 6 months of age: Ask a doctor
Inactive ingredients
ALUMINUM HYDROXIDE, BISABOLOL*,CAPRYLIC/CAPRIC TRIGLYCERIDE, COCONUT OIL*, DICAPRYLYL CARBONATE, FRAGANCE,HYDROGENATED COCONUT OIL**, KARANJATREE SEED EXTRACT, LIMONENE, LINALOOL,POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE,POLYGLYCERYL-3 DIISOSTEARATE ,POLYHYDROXYSTEARIC ACID, PROPANEDIOL,SESAME OIL*, STEARIC ACID, TOCOPHEROL,WATER.
| BIOSOLIS SUNSCREEN BROADSPECTRUM SPF50
titanium dioxide, zinc oxide cream |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - Pro Vera SA (375713286) |
| Registrant - Pro Vera SA (375713286) |
Revised: 1/2024
Document Id: 0e995eaf-1e87-306e-e063-6394a90a5d3f
Set id: ac9cf92e-b2d7-4f0c-a7f5-9179342496b7
Version: 4
Effective Time: 20240110
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.