PRIMA FLEUR HAND SANITIZER- ethyl alcohol gel
Prima Fleur Botanicals, Inc.
----------
Prima Fleur Botanicals Hand Sanitizer
Directions for use
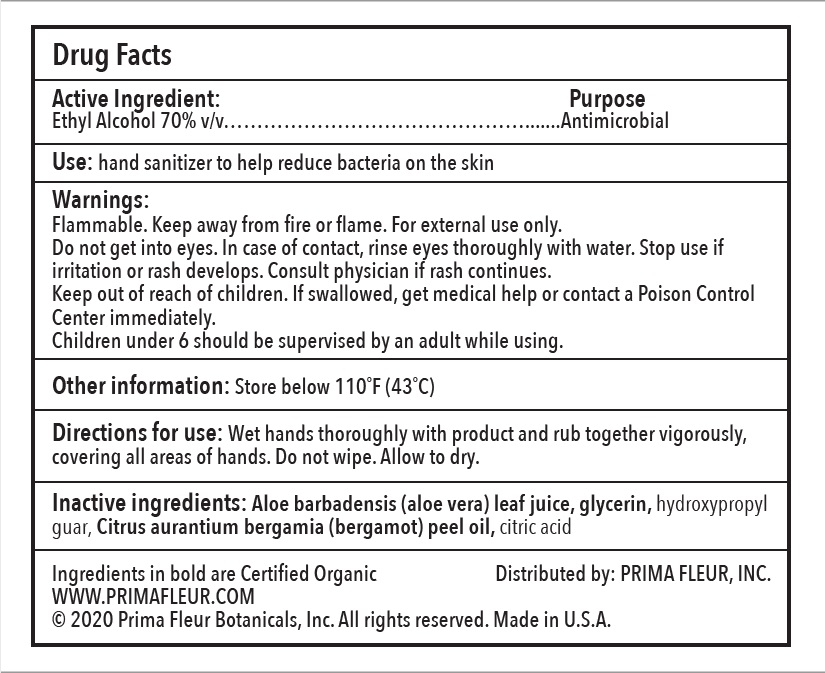
Warnings

Inactive Ingredients

Warnings

Use

Active Ingredients

Use

Prima Fleur Hand Sanitizer Gal front

Prima Fleur Hand Sanitizer 16oz front

Prima Fleur Hand Sanitizer 8oz front

Prima Fleur Hand Sanitizer 2oz front

PRIMA FLEUR HAND SANITIZER
ethyl alcohol gel |
| Product Information |
| Product Type | HUMAN OTC DRUG | Item Code (Source) | NDC: 71824-1702 |
| Route of Administration | CUTANEOUS |
|
| Active Ingredient/Active Moiety |
| Ingredient Name | Basis of Strength | Strength |
| ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) | ALCOHOL | 41.40297 mL in 59.15 mL |
|
| Inactive Ingredients |
| Ingredient Name | Strength |
| HYDROXYPROPYL GUAR (2500-4500 MPA.S AT 1%) (UNII: 3A1I7376TC) | |
| ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) | |
| GLYCERIN (UNII: PDC6A3C0OX) | |
| ALOE VERA LEAF (UNII: ZY81Z83H0X) | |
|
|
| Packaging |
| # | Item Code | Package Description | Marketing Start Date | Marketing End Date |
| 1 | NDC: 71824-1702-1 | 1 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product | 08/11/2020 | 03/01/2024 |
|
|
| Marketing Information |
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC Monograph Drug | 505G(a)(3) | 08/11/2020 | 03/01/2024 |
|
PRIMA FLEUR HAND SANITIZER
ethyl alcohol gel |
| Product Information |
| Product Type | HUMAN OTC DRUG | Item Code (Source) | NDC: 71824-1708 |
| Route of Administration | CUTANEOUS |
|
| Active Ingredient/Active Moiety |
| Ingredient Name | Basis of Strength | Strength |
| ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) | ALCOHOL | 165.6116 mL in 236.588 mL |
|
| Inactive Ingredients |
| Ingredient Name | Strength |
| HYDROXYPROPYL GUAR (2500-4500 MPA.S AT 1%) (UNII: 3A1I7376TC) | |
| ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) | |
| GLYCERIN (UNII: PDC6A3C0OX) | |
| ALOE VERA LEAF (UNII: ZY81Z83H0X) | |
|
|
| Packaging |
| # | Item Code | Package Description | Marketing Start Date | Marketing End Date |
| 1 | NDC: 71824-1708-1 | 1 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product | 08/11/2020 | 03/01/2024 |
|
|
| Marketing Information |
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC Monograph Drug | 505G(a)(3) | 08/11/2020 | 03/01/2024 |
|
PRIMA FLEUR HAND SANITIZER
ethyl alcohol gel |
| Product Information |
| Product Type | HUMAN OTC DRUG | Item Code (Source) | NDC: 71824-1716 |
| Route of Administration | CUTANEOUS |
|
| Active Ingredient/Active Moiety |
| Ingredient Name | Basis of Strength | Strength |
| ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) | ALCOHOL | 331.2232 mL in 473.176 mL |
|
| Inactive Ingredients |
| Ingredient Name | Strength |
| HYDROXYPROPYL GUAR (2500-4500 MPA.S AT 1%) (UNII: 3A1I7376TC) | |
| ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) | |
| GLYCERIN (UNII: PDC6A3C0OX) | |
| ALOE VERA LEAF (UNII: ZY81Z83H0X) | |
|
|
| Packaging |
| # | Item Code | Package Description | Marketing Start Date | Marketing End Date |
| 1 | NDC: 71824-1716-1 | 1 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product | 08/11/2020 | 03/01/2024 |
|
|
| Marketing Information |
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC Monograph Drug | 505G(a)(3) | 08/11/2020 | 03/01/2024 |
|
PRIMA FLEUR HAND SANITIZER
ethyl alcohol gel |
| Product Information |
| Product Type | HUMAN OTC DRUG | Item Code (Source) | NDC: 71824-1700 |
| Route of Administration | CUTANEOUS |
|
| Active Ingredient/Active Moiety |
| Ingredient Name | Basis of Strength | Strength |
| ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) | ALCOHOL | 2649.787 mL in 3785.41 mL |
|
| Inactive Ingredients |
| Ingredient Name | Strength |
| HYDROXYPROPYL GUAR (2500-4500 MPA.S AT 1%) (UNII: 3A1I7376TC) | |
| ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) | |
| GLYCERIN (UNII: PDC6A3C0OX) | |
| ALOE VERA LEAF (UNII: ZY81Z83H0X) | |
|
|
| Packaging |
| # | Item Code | Package Description | Marketing Start Date | Marketing End Date |
| 1 | NDC: 71824-1700-1 | 1 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product | 08/11/2020 | 03/01/2024 |
|
|
| Marketing Information |
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC Monograph Drug | 505G(a)(3) | 08/11/2020 | 03/01/2024 |
|
| Labeler - Prima Fleur Botanicals, Inc.
(825058308)
|
| Registrant - Prima Fleur Botanicals, Inc. (825058308) |
| Establishment |
| Name | Address | ID/FEI | Business Operations |
| Prima Fleur Botanicals, Inc. | | 825058308 | manufacture(71824-1700, 71824-1702, 71824-1708, 71824-1716) |




