VORAXAZE- glucarpidase injection, powder, for solution
Voraxaze by
Drug Labeling and Warnings
Voraxaze by is a Prescription medication manufactured, distributed, or labeled by BTG International Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use VORAXAZE® safely and effectively. See full prescribing information for VORAXAZE®.
VORAXAZE (glucarpidase) for injection, for intravenous use
Initial U.S. Approval: 2012INDICATIONS AND USAGE
VORAXAZE is a carboxypeptidase indicated to reduce toxic plasma methotrexate concentration (greater than 1 micromole per liter) in adult and pediatric patients with delayed methotrexate clearance (plasma methotrexate concentrations greater than 2 standard deviations of the mean methotrexate excretion curve specific for the dose of methotrexate administered) due to impaired renal function. (1)
Limitations of Use: VORAXAZE is not recommended for use in patients who exhibit the expected clearance and expected plasma methotrexate concentration. Reducing plasma methotrexate concentration in these patients may result in subtherapeutic exposure to methotrexate. (1)
DOSAGE AND ADMINISTRATION
- The recommended dosage of VORAXAZE is 50 Units per kilogram as a single intravenous injection over 5 minutes. (2.1).
- For the first 48 hours after the dose of VORAXAZE, administer the same leucovorin dose given prior to VORAXAZE. Administer leucovorin at least 2 hours before or 2 hours after the dose of VORAXAZE. (2.2)
- Beyond 48 hours after the dose of VORAXAZE, administer leucovorin based on the measured methotrexate concentration. Continue leucovorin until the methotrexate concentration has been maintained below the leucovorin treatment threshold for a minimum of 3 days. (2.2)
DOSAGE FORMS AND STRENGTHS
For Injection: 1,000 Units as a lyophilized powder in a single-dose vial for reconstitution (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Serious Hypersensitivity Reactions: Serious hypersensitivity reactions occurred. (5.1)
- Monitoring Methotrexate Concentration: Measure methotrexate concentrations within 48 hours following VORAXAZE administration using a chromatographic method; immunoassays are unreliable for samples collected within 48 hours following VORAXAZE administration. (5.2)
ADVERSE REACTIONS
The most common related adverse events (>1%) were paresthesia, flushing, nausea and/or vomiting, hypotension and headache. (6)
To report SUSPECTED ADVERSE REACTIONS, contact BTG at 877-377-3784 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Concomitant Use with Leucovorin Rescue
2.3 Preparation
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Serious Hypersensitivity Reactions
5.2 Interference with Immunoassay Measurements of Methotrexate
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Immunogenicity
7 DRUG INTERACTIONS
7.1 Effects of VORAXAZE on Leucovorin
7.2 Effect of VORAXAZE on Measurement of Methotrexate Concentration
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
VORAXAZE is indicated to reduce toxic plasma methotrexate concentration (greater than 1 micromole per liter) in adult and pediatric patients with delayed methotrexate clearance (plasma methotrexate concentrations greater than 2 standard deviations of the mean methotrexate excretion curve specific for the dose of methotrexate administered) due to impaired renal function.
Limitations of Use: VORAXAZE is not recommended for use in patients who exhibit the expected clearance and expected plasma methotrexate concentration. Reducing plasma methotrexate concentration in these patients may result in subtherapeutic exposure to methotrexate [see Clinical Studies (14)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dosage of VORAXAZE is 50 Units per kilogram (kg) as a single intravenous injection administered over 5 minutes. Flush intravenous line before and after administration.
2.2 Concomitant Use with Leucovorin Rescue
When administering VORAXAZE concomitantly with leucovorin, administer leucovorin at least 2 hours before or 2 hours after the VORAXAZE dose [see Drug Interactions (7.1)].
For the first 48 hours after a dose of VORAXAZE:
- Administer the same leucovorin dosage given prior to the VORAXAZE dose.
Beyond 48 hours after a dose of VORAXAZE:
- Determine the leucovorin dosage based on the measured methotrexate concentration. Do not discontinue leucovorin based on the determination of a single methotrexate concentration below the leucovorin rescue threshold.
- Continue leucovorin until the methotrexate concentration has been maintained below the leucovorin rescue threshold for a minimum of 3 days.
Continue intravenous hydration and urinary alkalinization as indicated.
When measuring methotrexate concentrations following a VORAXAZE dose, a chromatographic method is preferred over an immunoassay [see Warnings and Precautions (5.2)].
2.3 Preparation
Reconstitute the contents of the vial with 1 mL of 0.9% Sodium Chloride Injection, USP.
Roll and tilt the vial gently to mix. Do not shake.
Inspect the vial and discard VORAXAZE if the solution is not clear, colorless, and free of particulate matter.
Use reconstituted VORAXAZE immediately or store under refrigeration at 36° to 46°F (2° to 8°C) for up to 4 hours if not used immediately. VORAXAZE contains no preservative and is supplied as a single-dose vial. Discard any unused product.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Serious Hypersensitivity Reactions
Serious hypersensitivity reactions occurred in less than 1% of patients [see Adverse Reactions (6.1)].
5.2 Interference with Immunoassay Measurements of Methotrexate
DAMPA (4-deoxy-4-amino-N10- methylpteroic acid), an inactive metabolite of methotrexate formed following VORAXAZE administration, interferes with the measurement of methotrexate concentration using immunoassays. This interference results in an overestimation of the methotrexate concentration. Based on the half-life of DAMPA (about 9 hours), VORAXAZE may interfere with the measurement of methotrexate concentration for up to 48 hours following a VORAXAZE dose [see Clinical Pharmacology (12.1)].
When measuring methotrexate concentration following a VORAXAZE dose, a chromatographic method is preferred over an immunoassay.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Serious Hypersensitivity Reactions [Warnings and Precautions (5.1)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under controlled but widely varying conditions, adverse reaction rates observed in clinical trials of VORAXAZE cannot be directly compared to rates in the clinical trials of other drugs and may not reflect the rates observed in practice.
The evaluation of adverse reactions in patients who received VORAXAZE was confounded, because patients had toxic plasma methotrexate concentration due to prolonged methotrexate clearance, which is associated with myelosuppression, mucositis, acute hepatitis, and renal dysfunction and failure.
The safety of VORAXAZE is based on data from 290 patients who were enrolled in Study 1 or Study 2, two single-arm, open-label, multicenter studies conducted in patients who had markedly delayed methotrexate clearance due to impaired renal function. Patients with osteosarcoma were eligible for these studies if the plasma methotrexate concentration was >50 µmol/L at 24 hours, >5 µmol/L at 48 hours, or >2 standard deviations above the mean methotrexate elimination curve at least 12 hours after methotrexate administration; and there was a ≥2-fold increase in serum creatinine above baseline. All other patients were eligible for these studies if the plasma methotrexate concentration was >10 µmol/L more than 42 hours after the start of the methotrexate or the plasma methotrexate concentration was >2 standard deviations above the mean methotrexate excretion curve at least 12 hours following methotrexate; and the serum creatinine was >1.5 times the upper limit of normal (ULN) or the creatinine clearance (CLcr) was <60 mL/min at least 12 hours following methotrexate administration.
Safety data was available for 149 patients enrolled in Study 1. The protocol specified that patients with pre-VORAXAZE methotrexate concentration >100 μmol/L were to receive a second VORAXAZE dose 48 hours after the first dose; that patients continue receiving intravenous hydration, urinary alkalinization and leucovorin; and that leucovorin administration be adjusted to ensure that it was not administered within 2 hours before or after a VORAXAZE dose. VORAXAZE-related adverse reactions were collected on a flow sheet with a daily log of adverse reactions characterized as “glucarpidase toxicity”. Additional safety information was collected from clinical records submitted by treating physicians. This safety information was abstracted and categorized using the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 3. One (n=106) or 2 (n= 30) doses of VORAXAZE were administered; the number of doses was not specified for 13 patients. Doses ranged from 18 Units/kg to 98 Units/kg, with a median dose of 49 Units/kg. The median age was 18 years (1 month to 85 years); 63% were male; and the underlying malignancies were osteosarcoma/sarcomas in 32% and leukemia or lymphoma in 63% of patients.
Safety data was available for 141 patients enrolled in Study 2. The protocol did not specify the criterion for allowing patients to receive a second VORAXAZE dose. The protocol specified that patients continue receiving intravenous hydration, urinary alkalinization and leucovorin and that leucovorin administration be adjusted to ensure that it was not administered within 2 hours before or after VORAXAZE. VORAXAZE-related adverse reactions were collected and severity was graded according to NCI CTCAE version 3. One (n=122) or 2 (n= 18) doses of VORAXAZE were administered; the number of doses was not specified for 1 patient. Doses ranged from 6 Units/kg to 189 Units/kg, with a median dose of 50 Units/kg. The median age was 17 years (6 months to 85 years); 64% were male; and the underlying malignancies were osteogenic sarcoma in 32% and leukemia or lymphoma in 62% of patients.
Among the 290 patients, 8 deaths occurred within 30 days of VORAXAZE exposure that were not related to progressive disease.
The most common adverse reactions (reported in >1%) were paresthesia, flushing, and nausea and/or vomiting. Table 1 summarizes select adverse reactions; adverse reactions likely associated with toxic methotrexate plasma concentrations, such as hematological, renal and hepatic adverse reactions, were not included in this table.
Table 1: Select Adverse Reactions Occurring in Patients Receiving VORAXAZE in Study 1 and Study 2 1 This incidence includes the following terms: flushing, feeling hot, burning sensation.
2 One of these reactions was classified as Grade 3.Adverse Reaction VORAXAZE
N= 290Grades 1 and 22 (%) Paresthesia 2 Flushing1,2 2 Nausea/Vomiting 2 Headache 1 Hypotension 1 Blurred Vision <1 Diarrhea <1 Hypersensitivity <1 Hypertension <1 Rash <1 Throat irritation/Throat tightness <1 Tremor <1 6.2 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of incidence of antibodies in the studies described below with the incidence of antibodies in other studies or to other glucarpidase products may be misleading.
In clinical trials, 121 patients who received one (n=99), 2 (n=21), or 3 (n=1) doses of VORAXAZE were evaluated for anti-glucarpidase antibodies. Twenty-five of these 121 patients (21%) had detectable anti-glucarpidase antibodies following VORAXAZE administration, of which 19 received 1 dose of VORAXAZE and 6 received 2 doses of VORAXAZE. Antibody titers were determined using a bridging enzyme-linked immunosorbent assay (ELISA) for anti- glucarpidase antibodies.
Neutralizing antibodies were detected in 11 of the 25 patients who tested positive for anti- glucarpidase binding antibodies. Eight of these 11 patients had received a single dose of VORAXAZE; however, the development of neutralizing antibodies may be underreported due to lack of assay sensitivity.
-
7 DRUG INTERACTIONS
7.1 Effects of VORAXAZE on Leucovorin
VORAXAZE can decrease leucovorin concentration, which may decrease the effect of leucovorin rescue unless leucovorin is dosed as recommended [see Dosage and Administration (2.2), Clinical Pharmacology (12.3)].
VORAXAZE may also reduce the concentrations other folate analogs or folate analog metabolic inhibitors.
7.2 Effect of VORAXAZE on Measurement of Methotrexate Concentration
DAMPA (4-deoxy-4-amino-N10- methylpteroic acid), an inactive metabolite of methotrexate formed following VORAXAZE administration, interferes with the measurement of methotrexate concentration using immunoassays. This interference results in an overestimation of the methotrexate concentration. Based on the half-life of DAMPA, VORAXAZE may interfere with the measurement of methotrexate concentrations for approximately 48 hours following a VORAXAZE dose [see Warnings and Precautions (5.2)].
When measuring methotrexate concentration following a VORAXAZE dose, a chromatographic method is preferred over an immunoassay.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on VORAXAZE use in pregnant women or animal reproduction studies to evaluate for a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes.
VORAXAZE is administered in combination with methotrexate, which can cause embryo-fetal harm. Refer to methotrexate prescribing information for additional information.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of glucarpidase in human milk or its effects on the breastfed infant or on milk production.
VORAXAZE is administered in combination with methotrexate. Refer to methotrexate prescribing information for additional information.
8.4 Pediatric Use
The safety and effectiveness of VORAXAZE have been established in pediatric patients. Use of VORAXAZE for this indication is supported by evidence from a single-arm, open-label study in adult and pediatric patients 5 years of age and older with additional safety data in pediatric patients 1 to 17 years of age as described below.
Of the 22 patients in the efficacy dataset in Study 1, 12 were pediatric patients with ages ranging from 5 years to 16 years. Three of the 6 pediatric patients with a pre-VORAXAZE methotrexate concentration of 1 μmol/L to 50 μmol/L achieved a rapid and sustained clinically important reduction (RSCIR) in plasma methotrexate concentration, while none of the 6 pediatric patients with a pre-VORAXAZE methotrexate concentration >50 μmol/L achieved a RSCIR [see Clinical Studies (14)].
One-hundred forty-seven pediatric patients from 1 month to 17 years received VORAXAZE in Study 1 and Study 2 [see Adverse Reactions (6.1)]. No overall differences in safety were observed between these patients and adult patients.
8.5 Geriatric Use
Of the total number of 290 patients in clinical studies of VORAXAZE, 15% were 65 and over, while 4% were 75 and over. No overall differences in safety or effectiveness were observed between these patients and younger adult patients.
8.6 Renal Impairment
A study of the pharmacokinetics of glucarpidase in the absence of methotrexate in 4 subjects with severe renal impairment (CLcr <30 mL/min) showed that the mean pharmacokinetic parameters were similar to those observed in healthy subjects.
On this basis, no dose adjustment of VORAXAZE is recommended for patients with renal impairment [see Clinical Pharmacology (12.3)].
-
11 DESCRIPTION
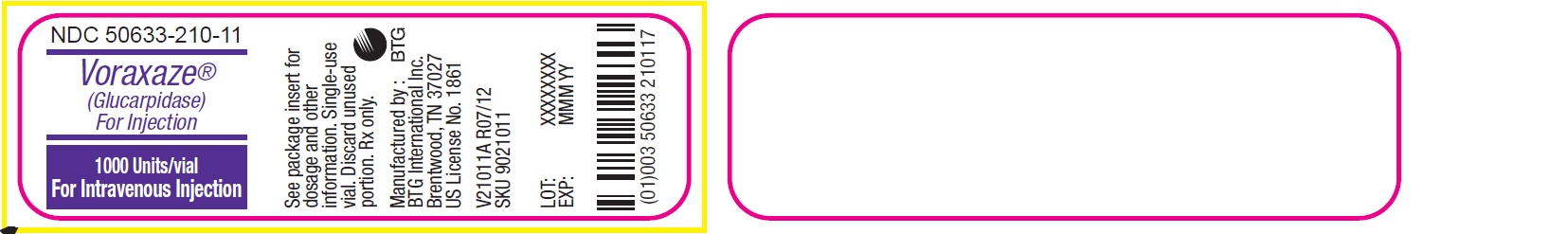
Glucarpidase is a carboxypeptidase produced by recombinant DNA technology in genetically modified Escherichia coli. Glucarpidase is a 390-amino acid homodimer protein with a molecular weight of 83 kDa. Each potency Unit corresponds to the enzymatic cleavage of 1 µmol/L of methotrexate per minute at 37°C.
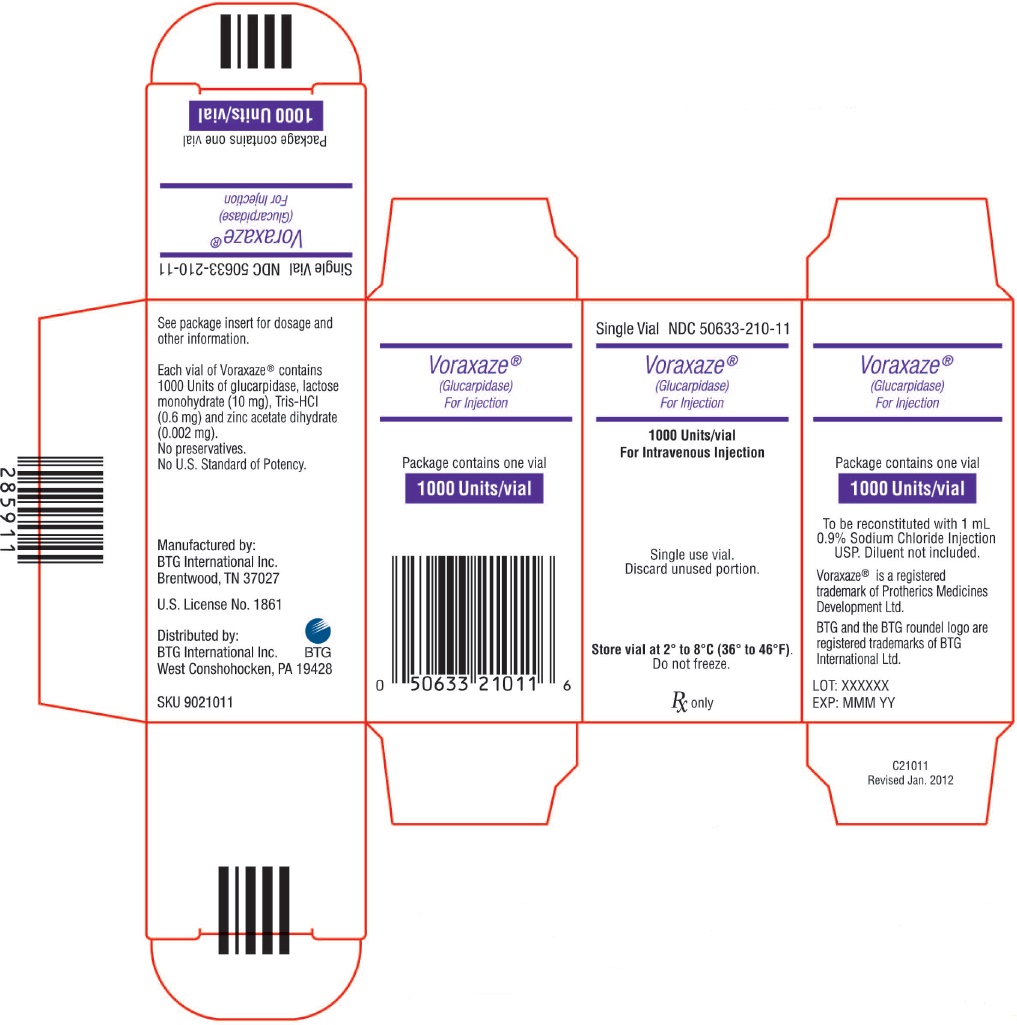
VORAXAZE (glucarpidase) for injection, for intravenous use is supplied as a sterile, preservative-free, white lyophilized powder in single-dose vials. Each vial contains 1,000 Units of glucarpidase, lactose monohydrate (10 mg), Tris-HCl (0.6 mg) and zinc acetate dihydrate (0.002 mg).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Glucarpidase is a recombinant bacterial enzyme that hydrolyzes the carboxyl- terminal glutamate residue from folic acid and classical antifolates such as methotrexate.
Glucarpidase converts methotrexate to its inactive metabolites 4-deoxy-4-amino-N10- methylpteroic acid (DAMPA) and glutamate. VORAXAZE provides an alternate non-renal pathway for methotrexate elimination in patients with renal dysfunction during high-dose methotrexate treatment.
12.2 Pharmacodynamics
Following administration of VORAXAZE 50 Units/kg to patients in Study 1, methotrexate concentration measured by a chromatographic method was reduced by ≥ 97% within 15 minutes in all 22 treatment-evaluable patients and was maintained at a > 95% reduction up to 8 days in 20 of the 22 patients [see Clinical Studies (14)].
12.3 Pharmacokinetics
The pharmacokinetics of glucarpidase in the absence of methotrexate were studied in 8 healthy subjects following VORAXAZE 50 Units/kg administered as an intravenous injection over 5 minutes. Serum glucarpidase activity levels were measured by an enzymatic assay and serum total glucarpidase concentrations were measured by ELISA. The mean Cmax was 3.3 μg/mL and the mean area under the curve (AUC0-INF) was 23.3 μg·h/mL. The pharmacokinetic parameters derived from the serum total glucarpidase concentrations were similar to those generated by serum glucarpidase activity levels except for elimination half-life as described below.
Distribution
The mean volume of distribution (Vd) was 3.6 L.
Elimination
Serum glucarpidase activity levels declined with a mean elimination half-life (t1/2) of 5.6 hours and serum total glucarpidase concentration declined with a mean elimination half-life of 9 hours. The mean systemic clearance (CL) was 7.5 mL/min.
Specific Populations
Patients with Renal Impairment
The pharmacokinetics of glucarpidase in the absence of methotrexate were studied in 4 subjects with severe renal impairment (CLcr <30 mL/min). Following a dose of VORAXAZE of 50 Units/kg, the mean pharmacokinetic parameters were similar to those observed in healthy subjects except for a longer half-life of 8.2 hours in subjects with severe renal impairment as compared to 5.6 hours in healthy subjects using an enzymatic assay to measure serum glucarpidase activity levels.
Drug Interaction Studies
In patients with cancer receiving high-dose methotrexate (≥1 g/m2) and leucovorin rescue, a VORAXAZE dose of 50 Units/kg administered intravenously 2 hours before leucovorin, reduced (6S)-leucovorin AUC0-3h by 33% and Cmax by 52% and reduced its active metabolite (6S)-5-methyltetrahydrofolate AUC0-3h by 92% and Cmax by 93% [see Drug Interactions (7.1)].
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
The efficacy of VORAXAZE was evaluated in a subset of 22 patients enrolled in Study 1 (NCT00001298), a single-arm, open-label study in patients who had markedly delayed methotrexate clearance (defined as more than 2 standard deviations greater than the mean excretion curve for methotrexate) due to impaired renal function. All patients received VORAXAZE 50 Units/kg as an intravenous injection over 5 minutes; those patients with pre-VORAXAZE methotrexate concentration >100 μmol/L were to receive a second dose of VORAXAZE 48 hours after the first dose. The protocol specified that patients continue receiving intravenous hydration, urinary alkalinization and leucovorin and that leucovorin administration be adjusted to ensure that it was not administered within 2 hours before or after VORAXAZE. These 22 patients had a pre-VORAXAZE methotrexate concentration >1 μmol/L and both pre- and post-treatment plasma samples available for determination of methotrexate concentration by a chromatographic method. The main outcome measure was the proportion of patients who achieved a rapid and sustained clinically important reduction (RSCIR) in plasma methotrexate concentration, defined as an attainment of plasma methotrexate concentration ≤1 μmol/L at 15 minutes that was sustained for up to 8 days following the initial injection.
The median age was 15.5 years (5 to 84 years); 59% were male; and the most common underlying cancers were osteogenic sarcoma (50%) and leukemia or lymphoma (45%).
Ten of the 22 patients achieved a RSCIR [45% (95% CI: 27%, 65%)]. Of the 12 patients who failed to achieve RSCIR, 5 patients (23%) attained a transient plasma methotrexate concentration ≤1 μmol/L. In these 5 patients, the median increase of plasma methotrexate concentration from their nadir was 1.4 μmol/L (0.3 to 2.5 μmol/L).
Table 2 summarizes the results of RSCIR and exploratory analyses following the first dose of VORAXAZE. An exploratory analysis in subgroups determined by pre-VORAXAZE methotrexate concentration suggests that the likelihood of attaining a RSCIR following the first VORAXAZE dose correlates with the pre-VORAXAZE methotrexate concentration. An additional exploratory analysis showed that all 9 patients with pre-VORAXAZE methotrexate concentration >50 μmol/L achieved >95% reduction in methotrexate concentration for up to 8 days following the first VORAXAZE dose although none of them achieved a RSCIR.
Table 2: Efficacy Results Following the First VORAXAZE Dose in Study 1 RSCIR: rapid and sustained clinically important reduction in methotrexate concentration. Pre-VORAXAZE
Methotrexate Concentration
(μmol/L)Patients
nPatients Achieving
RSCIR
n (%)Patients with >95% Rapid Reduction in
Methotrexate Concentration and
Maintained up to 8 Days
n (%)>1 22 10 (45%) 20 (91%) >1 to ≤50 13 10 (77%) 11 (85%) >50 to ≤100 2 0 2 (100%) >100 7 0 7 (100%) Lack of Efficacy with a Second Dose of VORAXAZE
Six of the 7 patients with pre-first dose VORAXAZE methotrexate concentration >100 μmol/L received a second VORAXAZE dose of 50 Units/kg administered 48 hours after the first dose. Among them, none of the 4 patients with pre-second dose VORAXAZE methotrexate concentration >1 μmol/L achieved a RSCIR. The remaining 2 patients achieved a RSCIR, but their pre-second dose VORAXAZE methotrexate concentration were already ≤1 μmol/L.
Deaths Attributable to Methotrexate Toxicity
There are no controlled trials comparing VORAXAZE and supportive care to supportive care alone in patients with toxic plasma methotrexate concentration due to impaired renal function; therefore, there are no data regarding the effect of VORAXAZE on survival or toxic deaths due to methotrexate. VORAXAZE did not prevent fatal methotrexate toxicity in 3% of patients in the safety population.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
VORAXAZE (glucarpidase) for injection is supplied as a sterile, preservative-free white lyophilized powder in an individually packaged glass single-dose vial closed with a bromo butyl elastomeric stopper and blue flip-off seal.
1,000 Units of glucarpidase per vial (1 vial per carton) NDC: 50633-210-11
Store VORAXAZE refrigerated at 36°F to 46°F (2°C to 8°C). Do not freeze. Do not use VORAXAZE after the expiration date on the vial.
-
17 PATIENT COUNSELING INFORMATION
Serious Hypersensitivity Reactions
Inform patients that hypersensitivity reactions, including potentially serious reactions, may occur following a dose of VORAXAZE and to immediately report any signs and symptoms of infusion reactions [see Warnings and Precautions (5.1)].
Administration
Inform patients of the importance of continued monitoring of plasma methotrexate concentration and renal function at the appropriate times after discharge from the hospital [see Warnings and Precautions (5.2)].
Manufactured and distributed by:
BTG International Inc.
West Conshohocken, PA 19428
U.S. license 1861
VORAXAZE® is a registered trademark of Protherics Medicines Development
Ltd. BTG and the BTG roundel logo are registered trademarks of BTG
International Ltd.
P21011D
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
VORAXAZE
glucarpidase injection, powder, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 50633-210 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLUCARPIDASE (UNII: 2GFP9BJD79) (GLUCARPIDASE - UNII:2GFP9BJD79) GLUCARPIDASE 1000 [USP'U] Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) 10 mg ZINC ACETATE (UNII: FM5526K07A) 0.002 mg TROMETHAMINE HYDROCHLORIDE (UNII: 383V75M34E) 0.6 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50633-210-11 1 in 1 CARTON 04/01/2012 1 1 in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125327 04/01/2012 Labeler - BTG International Inc. (617382395) Registrant - BTG International Inc. (617382395)
Trademark Results [Voraxaze]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VORAXAZE 85624617 4489059 Live/Registered |
Protherics Medicines Development Limited 2012-05-14 |
 VORAXAZE 85326368 4262065 Live/Registered |
Protherics Medicines Development Limited 2011-05-20 |
 VORAXAZE 78185379 3021666 Live/Registered |
PROTHERICS MEDICINES DEVELOPMENT LIMITED 2002-11-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.