Hydrating Duo by ULTRACEUTICALS PTY LIMITED / Nanophase Technologies Corporation Hydrating Duo
Hydrating Duo by
Drug Labeling and Warnings
Hydrating Duo by is a Otc medication manufactured, distributed, or labeled by ULTRACEUTICALS PTY LIMITED, Nanophase Technologies Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
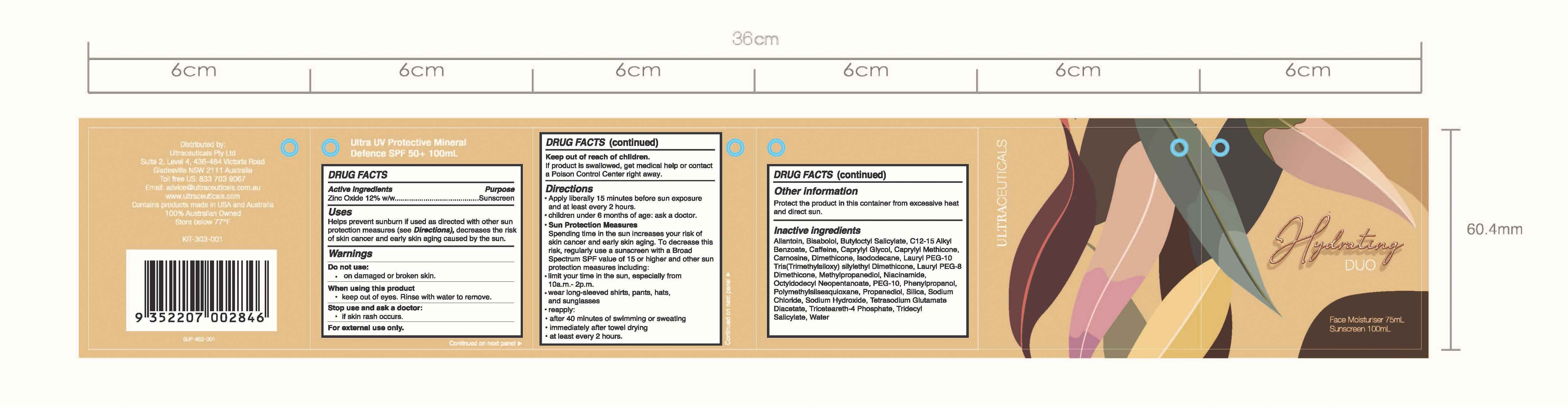
HYDRATING DUO- zinc oxide
ULTRACEUTICALS PTY LIMITED
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
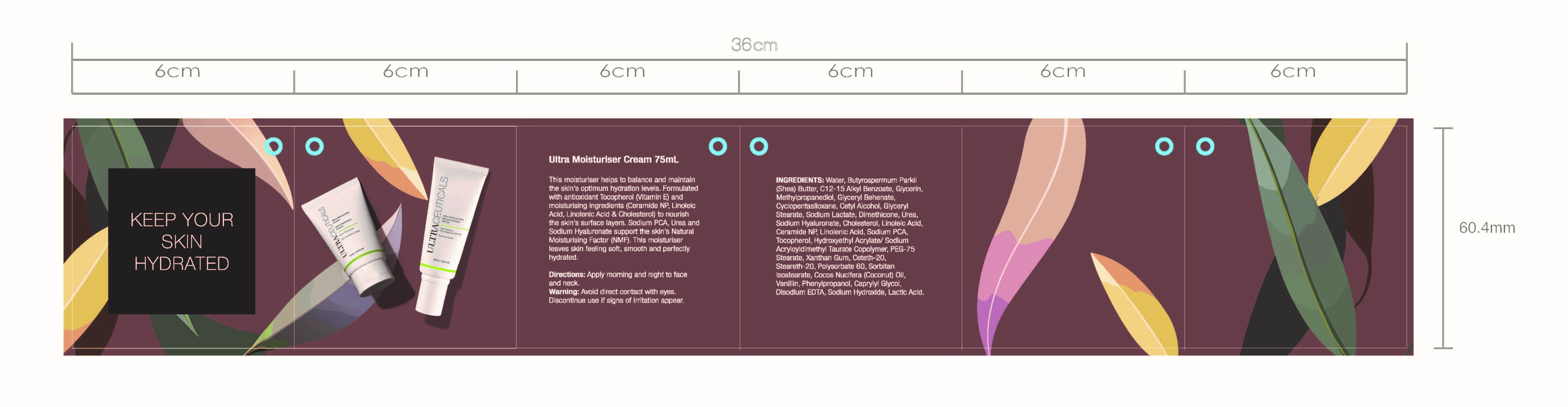
Hydrating Duo
Uses
Helps Prevent Sunburn
If used as directed with other sun protection measures (see
Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Keep out of reach of children. If product is swallowed, get medical help or contact a poison Control Center right away
Warnings
For external use only
Do not use on damaged or broken skin
When using this product keep out of eyes. Rinse with water to remove
Directions
Apply liberally 15 minutes before sun exposure and at least every 2 hours.
children under 6 months of age: ask a doctor.
Sun Protection Measures Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad-Spectrum SPF value of 15 or higher and other sun protection measures including:
limit your time in the sun, especially from 10 a.m. – 2 p.m.
wear long-sleeved shirts, pants, hats, and sunglasses
Reapply:
after 40 minutes of swimming or sweating
immediately after towel drying
at least every 2 hours
Allantoin, Bisabolol, Butyloctyl Salicylate, C12-15 Alkyl Benzoate, Caffeine, Caprylyl Glycol, Caprylyl Methicone, Carnosine, Dimethicone, Isododecane, Lauryl PEG-10 Tris(Trimethylsiloxy) Silylethyl Dimethicone, Lauryl PEG-8 Dimethicone, Methylpropanediol, Niacinamide, Octyldodecyl Neopentanoate, PEG-10, Phenylpropanol, Polymethylsilsesquioxane, Propanediol, Silica, Sodium Chloride, Sodium Hydroxide, Tetrasodium Glutamate Diacetate, Triceteareth-4 Phosphate, Tridecyl Salicylate, Water
| HYDRATING DUO
zinc oxide kit |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - ULTRACEUTICALS PTY LIMITED (744068995) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Nanophase Technologies Corporation | 623502044 | manufacture(10028-027) | |