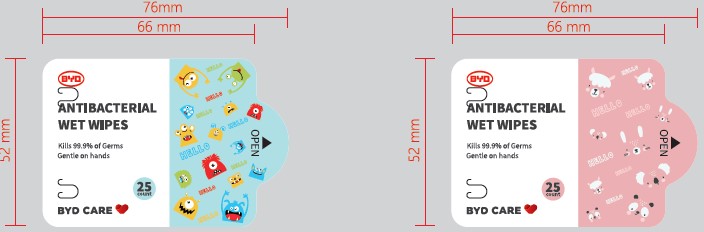
BYD Antibacterial Wet Wipes (Children) by BYD Automative Industry Co., Ltd.
BYD Antibacterial Wet Wipes (Children) by
Drug Labeling and Warnings
BYD Antibacterial Wet Wipes (Children) by is a Otc medication manufactured, distributed, or labeled by BYD Automative Industry Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
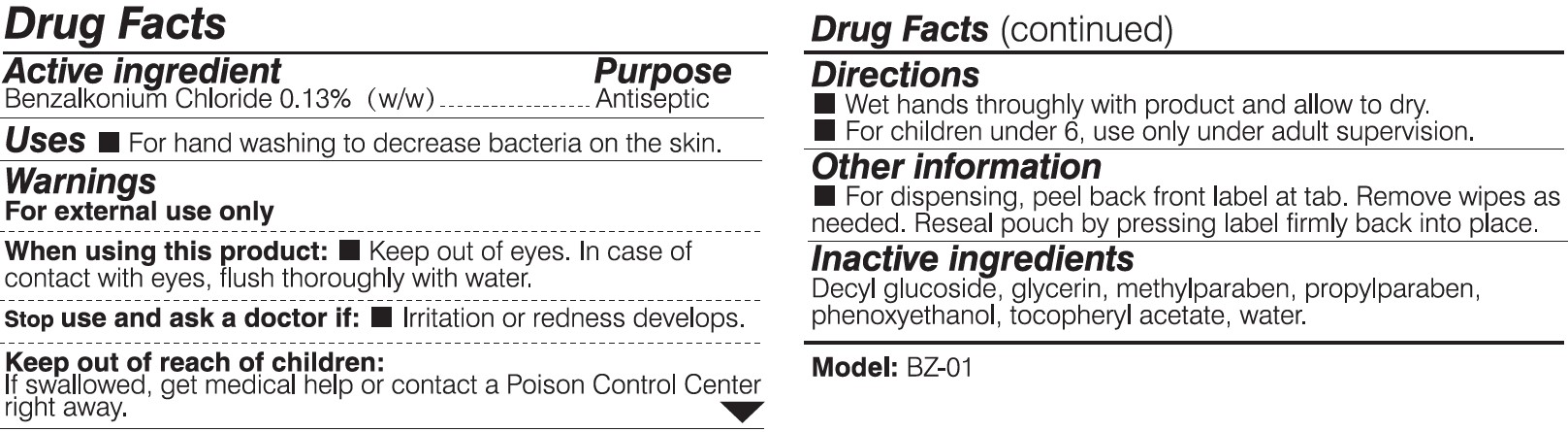
BYD ANTIBACTERIAL WET WIPES (CHILDREN)- disinfectant wipes cloth
BYD Automative Industry Co., Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Misc.
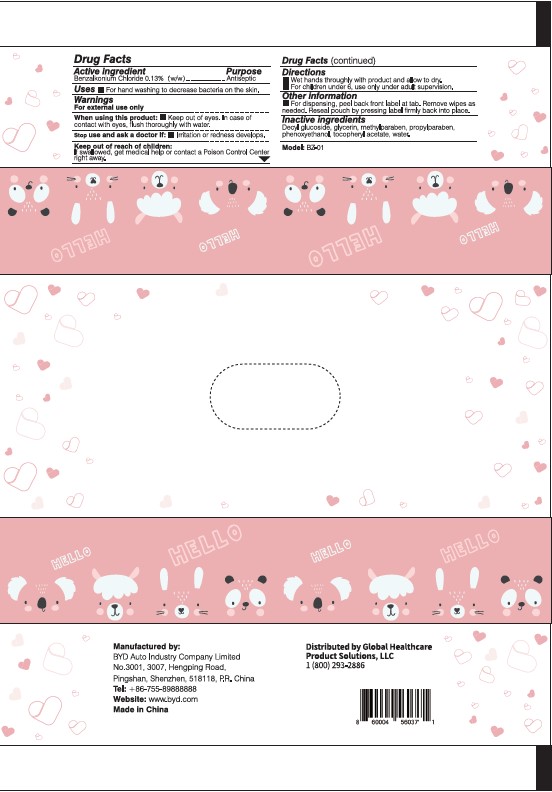
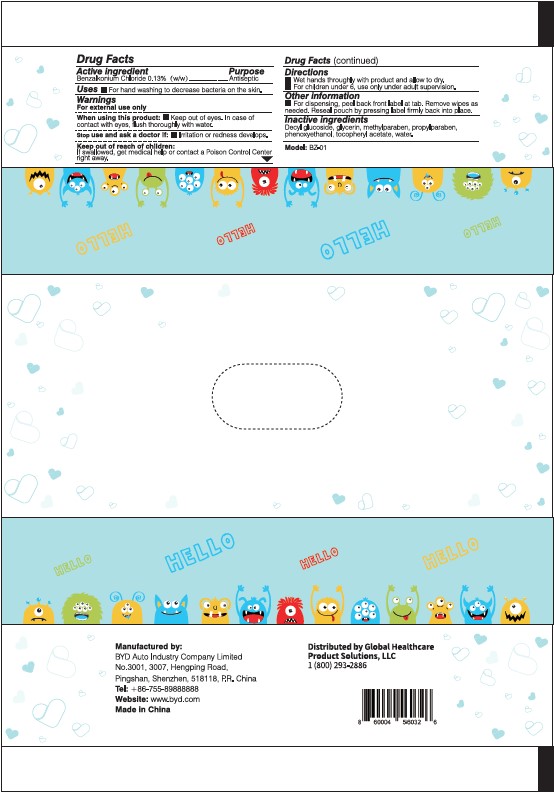
Manufactured by:
BYD Auto Industry Company Limited
No. 3001, 3007, Hengping Road,
Pingshan, Shenzhen, 518118, P.R. China
Tel: +86-755-89888888
Website: www.byd.com
Made in China
Distributed by Global Healthcare Product Solutions, LLC
1 (800) 293-2886


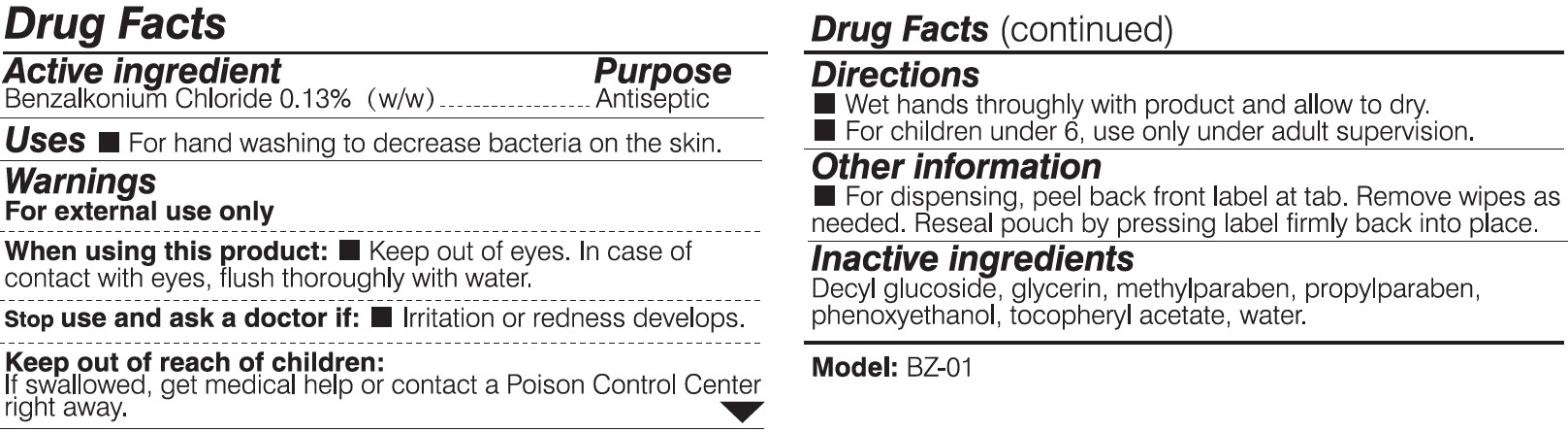
When using this product:
Keep out of eyes. In case of contact with eyes, flush thoroughly with water.
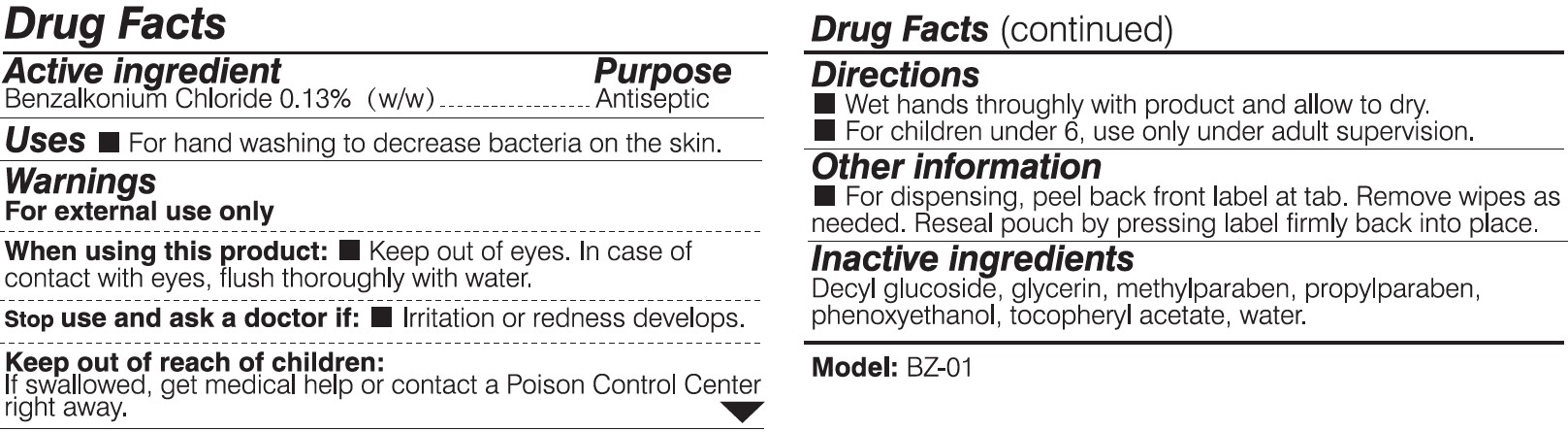
When using this product:
Keep out of eyes. In case of contact with eyes, flush thoroughly with water.
Keep out of reach of children:
If swallowed, get medical help or contact a Posion Control Center right away.
Directions:
Wet hands thoroughly with product and allow to dry.
For children under 6, use only under adult supervision.
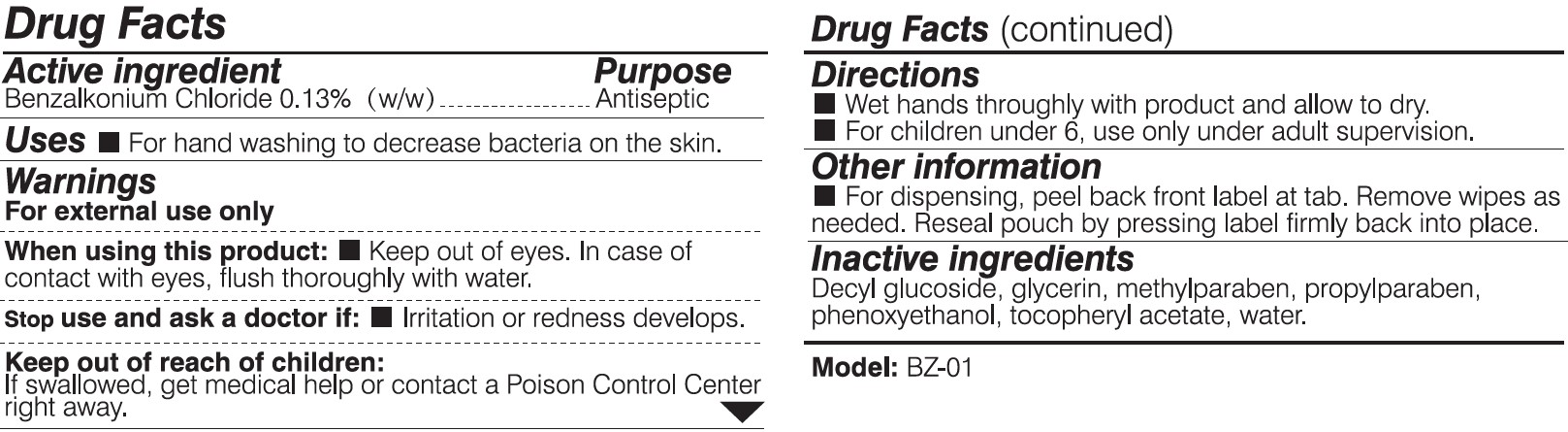
Other information:
For dispensing, peel back front label at tab. Remove wipes as needed. Reseal pouch by pressing label firmly back into place.
| BYD ANTIBACTERIAL WET WIPES (CHILDREN)
disinfectant wipes cloth |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| BYD ANTIBACTERIAL WET WIPES (CHILDREN)
disinfectant wipes cloth |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - BYD Automative Industry Co., Ltd. (545351723) |
| Registrant - BYD Automative Industry Co., Ltd. (545351723) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BYD Automative Industry Co., Ltd. | 545351723 | manufacture(75035-005, 75035-006) , label(75035-005, 75035-006) , pack(75035-005, 75035-006) | |