SeneGence SeneSun Hydrating Facial Sunscreen SPF 20
SeneGence SeneSun Hydrating Facial Sunscreen by
Drug Labeling and Warnings
SeneGence SeneSun Hydrating Facial Sunscreen by is a Otc medication manufactured, distributed, or labeled by SGII, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SENEGENCE SENESUN HYDRATING FACIAL SUNSCREEN SPF 20- avobenzone, homosalate, octisalate, octocrylene liquid
SGII, Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
SeneGence SeneSun Hydrating Facial Sunscreen SPF 20
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Following cleansing, squeeze a dime size amount of product onto fingers and gently massage into skin for an immediate boost of moisture, plus SPF protection.
For Sunscreen use:
- Apply generously evenly 15 minutes before sun exposure
- Reapply at least every 2 hours.
- Use a water-resistant sunscreen if swimming or sweating.
- Children under 6 months. Ask a doctor.
Sun Protection Measures:
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF of 15 or higher and other sun protection measures including:
-limit time in the sun, especially from 10 a.m.- 2 p.m.
-wear long-sleeved shirts, pants, hats and sunglasses.
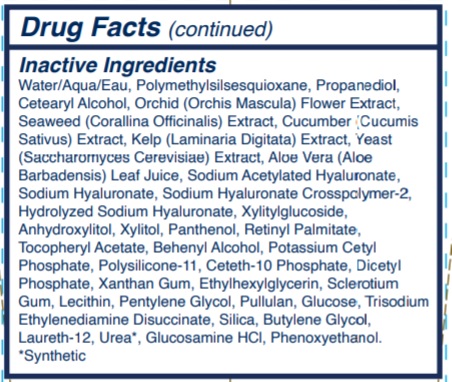
Inactive ingredients Water/Aqua/Eau, Polymethylsilsesquioxane, Propanediol, Cetearyl Alcohol, Orchid (Orchis Mascula) Flower Extract, Seaweed (Corallina Officinalis) Extract, Cucumber (Cucumis Sativus) Extract, Kelp (Laminaria Digitata) Extract, Yeast (Saccharomyces Cerevisiae) Extract, Aloe Vera (Aloe Barbadensis) Leaf Juice, Sodium Acetylated Hyaluronate, Sodium Hyaluronate, Sodium Hyaluronate Crosspolymer-2, Hydrolyzed Sodium Hyaluronate, Xylitylglucoside, Anhydroxylitol, Xylitol, Panthenol, Retinyl Palmitate, Tocopheryl Acetate, Behenyl Alcohol, Potassium Cetyl Phosphate, Polysilicone-11, Ceteth-10 Phosphate, Dicetyl Phosphate, Xanthan Gum, Ethylhexylglycerin, Sclerotium Gum, Lecithin, Pentylene Glycol, Pullulan, Glucose, Trisodium Ethylenediamine Disuccinate, Silica, Butylene Glycol, Laureth-12, Urea*, Glucosamine HCl, Phenoxyethanol.
*Synthetic
| SENEGENCE SENESUN HYDRATING FACIAL SUNSCREEN
SPF 20
avobenzone, homosalate, octisalate, octocrylene liquid |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - SGII, Inc (070096792) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.