NIVA-FOL- pyridoxine, folic acid, and cyanocobalamin tablet
Niva-Fol by
Drug Labeling and Warnings
Niva-Fol by is a Other medication manufactured, distributed, or labeled by Nivagen Pharmaceuticals, Inc., Natural Vitamins Laboratory Corp. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- HEALTH CLAIM
-
STATEMENT OF IDENTITY
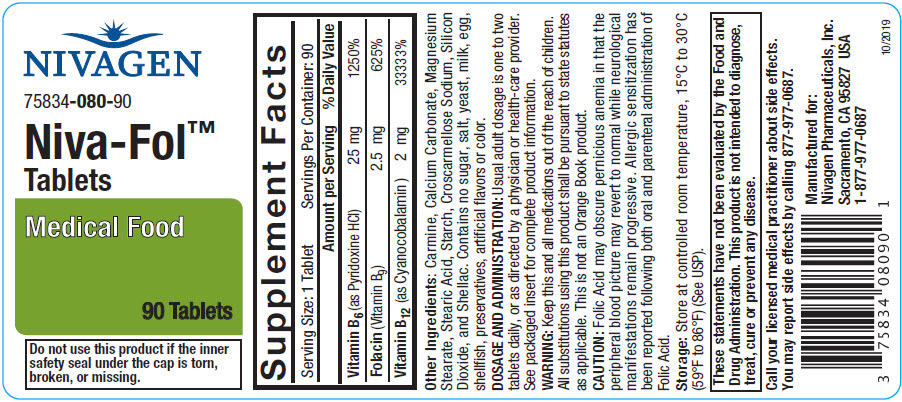
Each Niva-Fol™ Tablet contains:
Vitamin B6 (as Pyridoxine HCl) 25 mg Folacin (Folic Acid) 2.5 mg Vitamin B12 (as Cyanocobalamin) 2 mg Other Ingredients:
Carmine, Calcium Carbonate, Magnesium Stearate, Stearic Acid, Starch, Croscarmellose Sodium, Silicon Dioxide, and Shellac. Contains no sugar, salt, yeast, milk, egg, shellfish, preservatives, artificial flavors or color.
-
INDICATIONS AND USAGE
For the dietary management of individuals with distinct nutritional needs under a physician or health-care provider's supervision for hyperhomocysteinemia; with particular emphasis for individuals with or at risk for atherosclerotic vascular disease in the coronary1, peripheral2, or cerebral3 vessels, or vitamin B12 deficiency4. Niva-Fol™ Tablets are labeled as a medical food intended for use under active and ongoing medical supervision requiring medical care on a recurring basis for, among other things, instructions on the use of the medical food.
-
MEDICAL FOODS
Medical foods are Intended for the dietary management of a patient who, because of therapeutic or chronic medical needs, has limited or impaired capacity to ingest, digest, absorb, or metabolize ordinary foodstuffs or certain nutrients, or who has other special medically determined nutrient requirements, the dietary management of which cannot be achieved by the modification of a normal diet alone. Although a medical-food product is intended for use under the active and ongoing medical supervision, FDA does not require a prescription.
These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease. -
PRECAUTIONS
Folacin (folic acid) when administered as a single agent in doses above 0.1 mg daily, may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations remain progressive. The 2 mg of cyanocobalamin contained in Niva-Fol™ Tablets has been shown to provide an adequate amount of cyanocobalamin to address this precaution5. Unmetabolized folic acid has been shown in one study of 105 postmenopausal women (50-75 yrs) to have the potential to reduce natural killer cells' cytotoxicity, which may result in an impaired immune response6.
Cyanocobalamin should not be used in those with Leber's optic atrophy. Decreased levels of B12 have been associated with reduced ability to detoxify the cyanide in exposed individuals and cyanocobalamin may increase the risk of irreversible neurological damage from optic atrophy in those affected with the disorder. Hydroxocobalamin can aid in the detoxification of cyanide. This form of B12 is an acceptable form for B12 supplementation in those with this disorder.
Pregnant women and nursing mothers should only use 12 microgram doses of B12 (cyanocobalamin) from nutritional supplements. Doses higher than this should only be recommended by your physician. Administration of doses of vitamin B12 greater than 10 micrograms daily may produce a hematological response in those with anemia secondary to folate deficiency.
If pregnant, or planning to become pregnant or are currently breast-feeding please contact your physician, or health-care provider before using or continuing use.
-
ADVERSE REACTIONS
Allergic sensitization has been reported following both oral and parenteral administration of folacin (folic acid). Paresthesia, somnolence, nausea and headaches have been reported with pyridoxine. Mild transient diarrhea, polycythemia vera, itching, transitory exanthema, and the feeling of swelling of the entire body has been associated with cyanocobalamin.
- CONTRAINDICATIONS
-
DRUG INTERACTIONS
Pyridoxine supplements should not be given to patients receiving the drug levodopa, because the action of levodopa is antagonized by pyridoxine. However, pyridoxine may be used concurrently in patients receiving a preparation containing both carbidopa and levodopa. Concurrent use of phenytoin and folacin (folic acid) may result in decreased phenytoin effectiveness.
- PATIENT INFORMATION
- DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
Niva-Fol™ Tablets are available as an oval, coated tablet, debossed "N080". Supplied in bottles of 90 Tablets, 75834-080-90
Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F).
See USP Controlled Room Temperature.
Protect from light and moisture.
Dispense in a tight, light-resistant container with a child-resistant closure as defined in the USP/NF.
- SAFE HANDLING WARNING
- Medical Food
-
References
1, 2 Eikelboom JW, Lonn Eva, Genest Jr Jaques, Hankey Graeme, Yusuf Salim: Homocysteine and Cardiovascular Disease: A Critical Review of the Epidemiologic Evidence. Ann Intern Med. 1999; 131:363-375.
3 The Homocysteine Studies Collaboration: Homocysteine and Risk of lschemic Heart Disease and Stroke. JAMA 2002; Vol 288, No. 16:2015-2022.
4 Refsum Helga, Smith A. David, Ueland Per M, Nexo Ebba, Clarke Robert, McPartlin Joseph, Johnston Carole, Engbaek Frode, Schneede Jorn, McPartlin Catherine, and Scott John M.: Facts and Recommendations about Total Homocysteine Determinations: An Expert Opinion. Clinical Chemistry 2004; 50(1):3-32.
5 Kuzminski AM, Del Giacco EJ, Allen RH, et al: Effective Treatment of Cobalamin Deficiency with Oral Cobalamin. Blood 1998; 92:1191-1198.
6 Troen AM, Mitchell B, Sorensen B, Wener MH, Johnston A, Wood B, Selhub J, McTiernan A, Yasui Y, Oral E, Potter JD, and Ulrich CM: Unmetabolized Folic Acid in Plasma is Associated with Reduced Natural Killer Cell Cytotoxicity among Postmenopausal Women. Journal of Nutrition 2006 Jan; 136(1): 189-194.
Rev. 10/19
- PRINCIPAL DISPLAY PANEL - 90 Tablet Bottle Label
-
INGREDIENTS AND APPEARANCE
NIVA-FOL
pyridoxine, folic acid, and cyanocobalamin tabletProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:75834-080 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Pyridoxine Hydrochloride (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) Pyridoxine Hydrochloride 25 mg Folic Acid (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) Folic Acid 2.5 mg Cyanocobalamin (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) Cyanocobalamin 2 mg Inactive Ingredients Ingredient Name Strength Calcium Carbonate (UNII: H0G9379FGK) Magnesium Stearate (UNII: 70097M6I30) Stearic Acid (UNII: 4ELV7Z65AP) Croscarmellose Sodium (UNII: M28OL1HH48) Silicon Dioxide (UNII: ETJ7Z6XBU4) Shellac (UNII: 46N107B71O) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:75834-080-90 90 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 09/10/2021 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color scoring 1 shape size (solid drugs) 60 mm imprint Labeler - Nivagen Pharmaceuticals, Inc. (052032418) Establishment Name Address ID/FEI Business Operations Natural Vitamins Laboratory Corp 928327774 MANUFACTURE(758324-080)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.