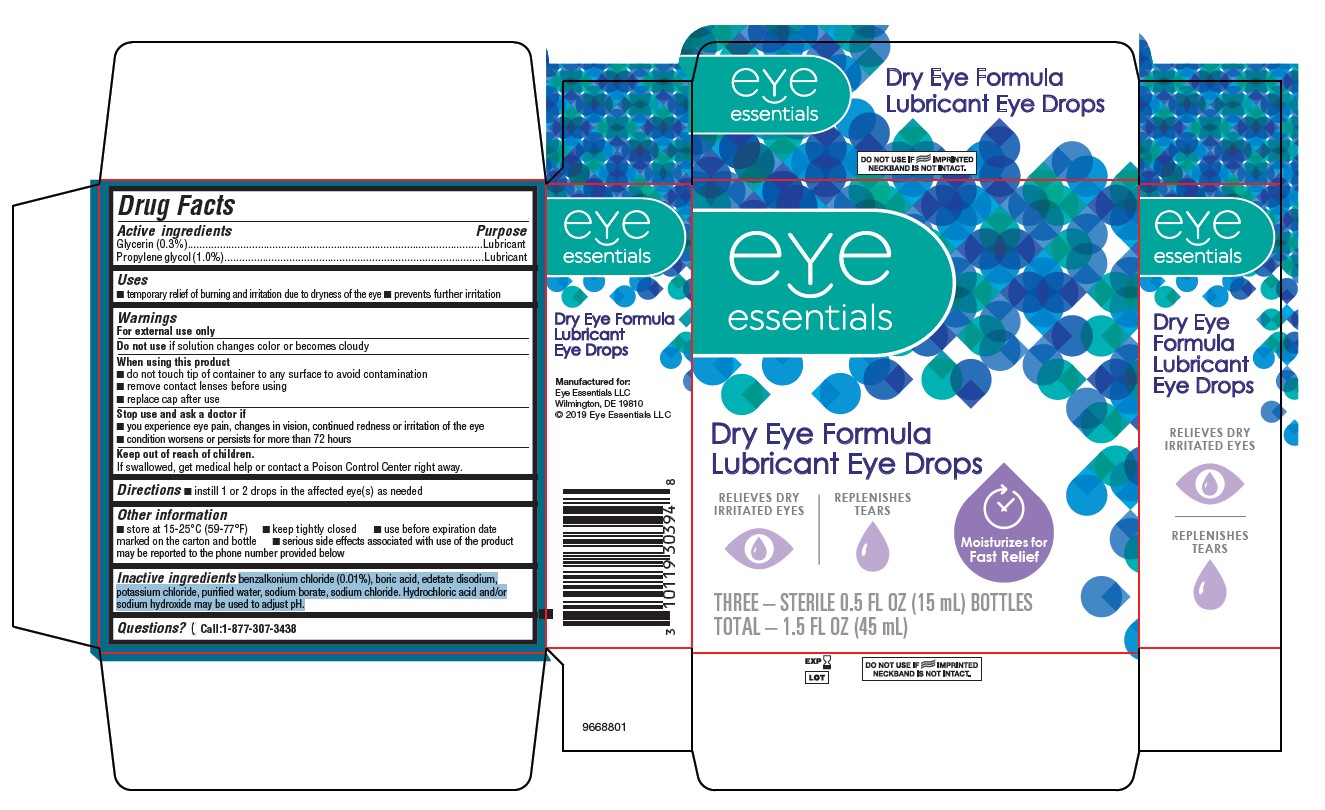
Dry Eye Formula Lubricant Eye Drops by Eye Essentials LLC / Bausch & Lomb Incorporated Drug Facts
Dry Eye Formula Lubricant Eye Drops by
Drug Labeling and Warnings
Dry Eye Formula Lubricant Eye Drops by is a Otc medication manufactured, distributed, or labeled by Eye Essentials LLC, Bausch & Lomb Incorporated. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
DRY EYE FORMULA LUBRICANT EYE DROPS- glycerin propylene glycol solution/ drops
Eye Essentials LLC
----------
Drug Facts
Uses
- temporary relief of burning and irritation due to dryness of the eye
- prevents further irritation
Warnings
For external use only
Do not useif solution changes color or becomes cloudy
When using this product
- do not touch tip of container to any surface to avoid contamination
- remove contact lenses before using
- replace cap after use
Stop use and ask a doctor if
- you experience eye pain, changes in vision, continued redness or irritation of the eye
- condition worsens or persists for more than 72 hours
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Other information
- store at 15-25°C (59-77°F)
- keep tightly closed
- use before expiration date marked on the carton and bottle
- serious side effects associated with use of the product may be reported to the phone number provided below
| DRY EYE FORMULA LUBRICANT EYE DROPS
glycerin propylene glycol solution/ drops |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Eye Essentials LLC (117007610) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bausch & Lomb Incorporated | 114406598 | manufacture(72985-001) | |
Revised: 7/2024
Document Id: 1cbfb27f-bfc9-3b19-e063-6394a90aeca4
Set id: acd6025c-1b5e-4b04-9d26-3305659b73fc
Version: 5
Effective Time: 20240708
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.