RIVFLOZA- nedosiran injection, solution
RIVFLOZA by
Drug Labeling and Warnings
RIVFLOZA by is a Prescription medication manufactured, distributed, or labeled by Novo Nordisk. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use RIVFLOZA safely and effectively. See full prescribing information for RIVFLOZA.
RIVFLOZA® (nedosiran) injection, for subcutaneous use
Initial U.S. Approval: 2023INDICATIONS AND USAGE
RIVFLOZA is an LDHA-directed small interfering RNA indicated to lower urinary oxalate levels in children 2 years of age and older and adults with primary hyperoxaluria type 1 (PH1) and relatively preserved kidney function, e.g., eGFR ≥30 mL/min/1.73 m2. (1)
DOSAGE AND ADMINISTRATION
The recommended dosage is shown below and is administered subcutaneously once monthly. (2.1)
Body weight
Less than 39 kg
39 kg to less than 50 kg
50 kg and above
Age 2 to less than 12 years
3.3 mg/kg
128 mg
160 mg
Age 12 years and older
128 mg
160 mg
See full Prescribing Information for important administration instructions. (2.2)
DOSAGE FORMS AND STRENGTHS
RIVFLOZA Injection 160 mg/mL is a clear, colorless-to-yellow solution available as follows:
- 80 mg/0.5 mL single-dose vial
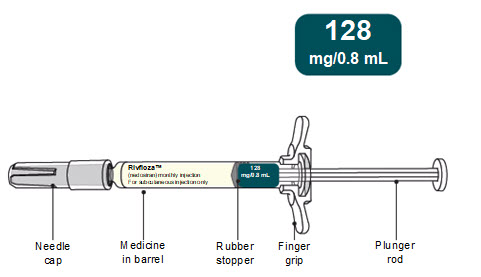
- 128 mg/0.8 mL single-dose Pre-filled Syringe
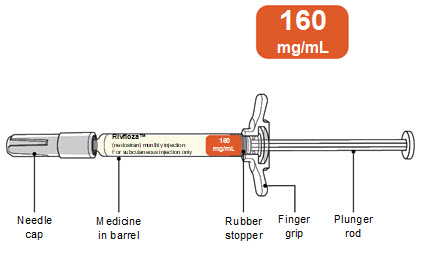
- 160 mg/ mL single-dose Pre-filled Syringe (3)
CONTRAINDICATIONS
None. (4)
ADVERSE REACTIONS
Most common adverse reactions (reported in ≥20% of patients) are injection site reactions. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Novo Nordisk Inc. at 1-844-906-5099 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 PHYOX2
14.2 PHYOX8
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
RIVFLOZA is administered subcutaneously once monthly at the recommended doses shown in Table 1.
Dosing is based on actual body weight.
Table 1: RIVFLOZA Dose Regimen in Adults and Pediatric Patients (2 years of age and older)
Body weight
Less than 39 kg
39 kg to less than 50 kg
50 kg and above
Age 2 to less than 12 years
3.3 mg/kg
128 mg
160 mg
Age 12 years and older
128 mg
160 mg
Missed Dose
If a planned dose is missed, administer RIVFLOZA as soon as possible. If the planned dose is missed by more than 7 days, administer RIVFLOZA as soon as possible and resume monthly dosing from the most recently administered dose.
2.2 Administration Instructions
Pre-filled syringe: A healthcare provider, caregiver, or patient 12 years of age and older may inject RIVFLOZA using the pre-filled syringe. In pediatric patients 2 to less than 12 years of age who weigh ≥39 kg, a healthcare provider or caregiver may inject RIVFLOZA using the pre-filled syringe.
Vials: RIVFLOZA vials are intended for use under the guidance and supervision of a healthcare provider. Adult patients or caregivers may administer RIVFLOZA after proper training in preparing RIVFLOZA vials for administration, if a healthcare provider determines that it is appropriate, and with medical follow-up as necessary.
Administer RIVFLOZA by subcutaneous injection to the abdomen (at least 2 inches from the navel) or the upper thigh. Do not inject into a vein or into scarred or bruised skin.
Inspect visually for particulate matter and discoloration prior to injection. RIVFLOZA should be colorless-to-yellow and particle free. If the solution is cloudy or contains particulate matter, do not use.
Instructions for delivering the dosage are provided in the Instructions for Use leaflets enclosed with the RIVFLOZA Pre-filled Syringe and single-dose vial.
Discard the unused portion of the drug.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of RIVFLOZA has been evaluated in one placebo-controlled clinical trial (PHYOX2) and one open-label extension study (PHYOX3). Across these studies, 29 adults and 12 children with PH1 have been treated with RIVFLOZA. Patients with PH1 in these studies ranged in age from 9 to 46 years at first dose. The median duration of exposure was approximately 15 months (range 1-29 months). Overall, 38 patients with PH1 were treated for at least 6 months, 24 patients for at least 12 months, and 16 patients for at least 18 months.
In the randomized, placebo-controlled, double-blind PHYOX2 trial in pediatric and adult patients 9 to 46 years of age, 18 patients with PH1 received RIVFLOZA and 11 patients received placebo. Of the 18 patients treated with RIVFLOZA, 17 patients received ≥5 months of active treatment. The most common adverse reactions were injection site reactions, which were reported in 7 patients with PH1 (39%) on RIVFLOZA as compared to no patients on placebo. Injection site reactions included erythema, pain, bruising, and rash and were generally mild and did not lead to discontinuation of treatment.
In the single-arm extension study (PHYOX3) that included 40 patients with PH1, additional injection site reactions included atrophy in 1 patient (3%).
The safety of RIVFLOZA has additionally been evaluated in one single-arm clinical study (PHYOX8) in 15 pediatric patients 2 to less than 12 years of age with PH1 and an eGFR >30 mL/min/1.73 m2. Injection site reactions were reported in 2 patients (13%). Overall, the RIVFLOZA safety profile was similar to that seen in PHYOX2.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from reports of pregnancy in clinical trials with RIVFLOZA are insufficient to evaluate for a drug-associated risk of major birth defects, miscarriage or other adverse maternal or fetal outcomes.
In animal reproduction studies, no adverse developmental effects were observed when nedosiran was administered to pregnant mice at doses up to approximately 58 times the maximum recommended human dose (MRHD) of 160 mg nedosiran (equivalent to 170 mg nedosiran sodium) per dose, based on body surface area (BSA) or upon administration of a mouse-specific (pharmacologically active) analog. Subcutaneous administration of nedosiran to pregnant rabbits during the period of organogenesis at doses approximating the MRHD resulted in increased fetal loss in the presence of maternal toxicity. Adverse developmental outcomes (fetal cardiovascular and skeletal malformations) were observed at a dose approximately 2 times the MRHD (see Data). Nedosiran is not pharmacologically active in rabbits or mice. The cause for the embryo-fetal toxicities observed in rabbits remains unclear.
The estimated background risk of major birth defects and miscarriage in the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
In mice, subcutaneous administration of nedosiran at doses up to 2000 mg/kg/dose (approximately 58 times the MRHD based on BSA) or a mouse-specific (pharmacologically active) analog (10 mg/kg/dose) during organogenesis (dosing on gestation days 6, 8, 10, 12, and 14 for nedosiran; gestation days 3 and 10 for the analog) did not have adverse effects on embryo-fetal development.
Subcutaneous administration of nedosiran (0, 2, 6 or 20 mg/kg/dose) to pregnant rabbits during organogenesis (dosing on gestation days 7, 9, 11, 13, 15, 17, and 19) resulted in maternal toxicity on the basis of body weight loss of up to 6.5% following the first dose in the 6 and 20 mg/kg/dose groups. Higher post-implantation loss and lower numbers of live fetuses occurred at ≥6 mg/kg/dose (exposures equivalent to the MRHD based on BSA), and fetal cardiovascular and skeletal malformations occurred at the 20 mg/kg/dose (2 times the MRHD based on BSA). At the 2 mg/kg/dose, which is below the MRHD, no adverse findings were seen.
In a pre- and postnatal study in mice, subcutaneous administration of nedosiran (0, 250, 500, or 1000 mg/kg/dose) or a mouse-specific (pharmacologically active) analog (10 mg/kg/dose) from implantation (dosing on gestational days 6, 8, 10, 12, 14, 16) to weaning (dosing on lactation days 1, 8, 15, 20) did not have adverse effects on the growth, viability, development and reproductive performance of the offspring.
8.2 Lactation
Risk Summary
There are no data on the presence of RIVFLOZA in human or animal milk, the effects on the breastfed child, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for RIVFLOZA and any potential adverse effects on the breastfed infant from RIVFLOZA or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of RIVFLOZA have been established in pediatric patients aged 2 years and older. Use of RIVFLOZA in these age groups is supported by evidence from an adequate and well-controlled trial in adult and pediatric patients 9 years of age and older (PHYOX2), and a single-arm study in pediatric patients 2 to less than 12 years of age (PHYOX8) [see Clinical Studies (14)].
The safety and effectiveness of RIVFLOZA in patients younger than 2 years of age have not been established.
8.5 Geriatric Use
Clinical studies of RIVFLOZA did not include patients aged 65 and over to determine whether they respond differently from younger patients. No dose adjustment is recommended in patients ≥65 years old [see Clinical Pharmacology (12.3)].
8.6 Hepatic Impairment
No dose adjustment of RIVFLOZA is recommended for patients with mild hepatic impairment (total bilirubin ≤ upper limit of normal [ULN] and aspartate aminotransferase [AST] > ULN or total bilirubin >1 to 1.5 times ULN and any AST).
RIVFLOZA has not been studied in patients with moderate or severe hepatic impairment (total bilirubin >1.5 ULN with any AST) [see Clinical Pharmacology (12.3)].
8.7 Renal Impairment
No dose adjustment is recommended in patients with an estimated glomerular filtration rate (eGFR) of ≥30 mL/min/1.73 m2 [see Clinical Pharmacology (12.3)].
RIVFLOZA has not been studied in PH1 patients with severe renal impairment (eGFR <30 mL/min/1.73 m2).
-
11 DESCRIPTION
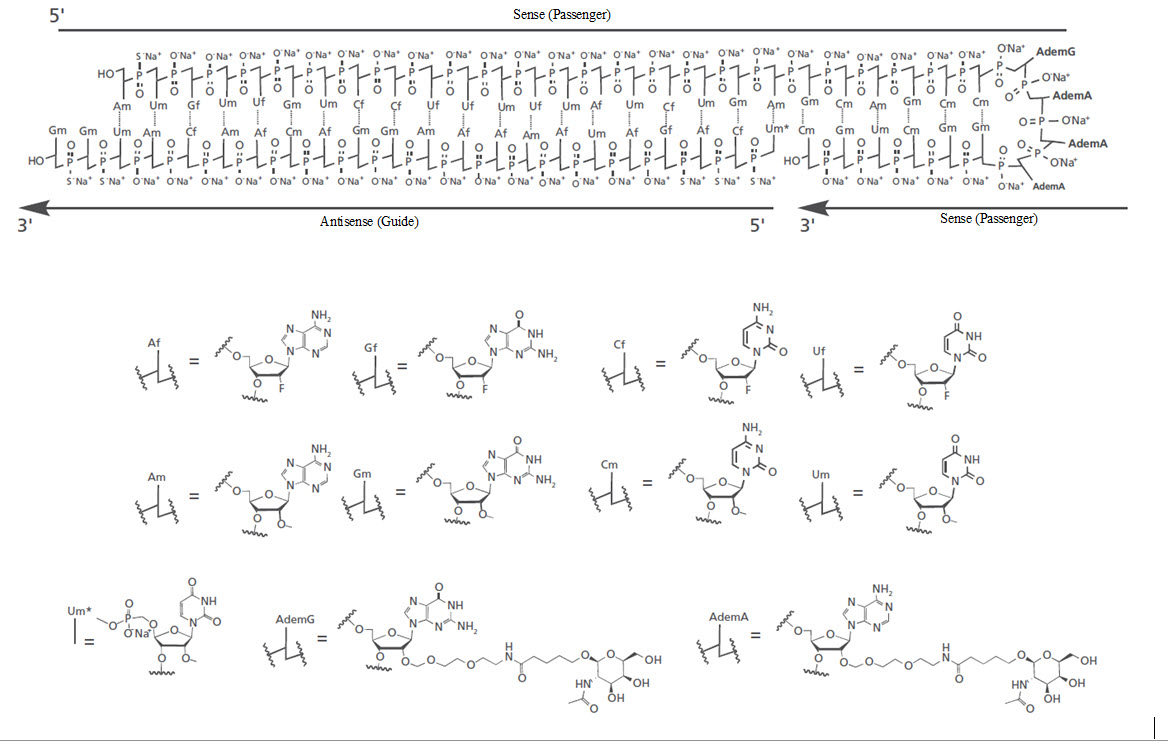
RIVFLOZA injection contains nedosiran, a double-stranded small interfering RNA (siRNA) with four covalently attached N-acetyl-D-galactosamine (GalNAc) residues. Nedosiran targets lactate dehydrogenase A (LDHA) in hepatocytes via GalNAc-mediated delivery.
The structural formula of the nedosiran sodium drug substance is presented below:
The molecular formula of nedosiran sodium is C662H808F19N231O413P57S6Na57 with a molecular weight of 22,238 Da. Nedosiran sodium is freely soluble in water.
RIVFLOZA Pre-filled Syringe is supplied as a clear, sterile, preservative-free, colorless‑to‑yellow solution for subcutaneous injection containing either the equivalent of 160 mg (present as 170 mg nedosiran sodium salt) nedosiran in 1 mL or the equivalent of 128 mg (present as 136 mg nedosiran sodium salt) nedosiran in 0.8 mL of water for injection and sodium hydroxide and/or hydrochloric acid to adjust the pH to ~7.2.
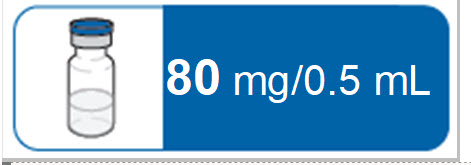
RIVFLOZA vial is supplied as a clear, sterile, preservative-free, colorless-to-yellow solution for subcutaneous injection containing the equivalent of 80 mg (present as 85 mg nedosiran sodium salt) nedosiran in 0.5 mL of water for injection and sodium hydroxide and/or hydrochloric acid to adjust the pH to ~7.2.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Nedosiran is a double-stranded siRNA, conjugated to GalNAc aminosugar residues. After subcutaneous administration, the GalNAc-conjugated sugars bind to asialoglycoprotein receptors (ASGPR) to deliver nedosiran to hepatocytes.
Nedosiran reduces levels of hepatic lactate dehydrogenase (LDH) via the degradation of LDHA messenger ribonucleic acid (mRNA) in hepatocytes through RNA interference. The reduction of hepatic LDH by nedosiran reduces the production of oxalate by the liver, thereby reducing subsequent oxalate burden.
12.2 Pharmacodynamics
The pharmacodynamic effects of RIVFLOZA were evaluated after single-dose and monthly-dose administration in patients with PH1. Dose-dependent reductions in urinary oxalate were observed in the single-dose range of 1.5 mg/kg to 6.0 mg/kg. With the recommended monthly dose regimen of RIVFLOZA, onset of effect was observed at the first measurement (30 days after the first dose) and the effect persisted with continued monthly dosing [see Clinical Studies (14.1)].
Cardiac Electrophysiology
At the recommended dose, RIVFLOZA does not lead to clinically relevant QT interval prolongation.
12.3 Pharmacokinetics
The pharmacokinetic (PK) properties of RIVFLOZA were evaluated following administration of single and multiple dosages in patients with PH1 or PH2 as summarized in Table 2.
Table 2: Pharmacokinetic Parameters of Nedosiran
Nedosiran
General Information
Steady State Exposure
Cmax [Mean (%CV)]
844 (44) ng/mL
AUC0-last [Mean (%CV)]
- 13600 (36) ng*h/mL
Dose Proportionality
Nedosiran exhibited a dose-proportional increase in plasma exposure following single subcutaneous doses from 1.5 to 6.0 mg/kg.
Nedosiran exhibited time-independent pharmacokinetics with multiple doses of 160 mg once monthly (body weight ≥50 kg), 128 mg once monthly (body weight <50 kg), or 3.3 mg/kg once monthly in the age range of 6 to 11 years.
Accumulation
No accumulation of nedosiran was observed in plasma following repeated monthly dosing.
Absorption
Tmax [Median (Range)]
6 (2 to 12) hours
Distributiona
Estimated Vz/F
126 L
Protein Binding
85.6%
Elimination
Half-Life (Mean (%CV)])
15 (68) hours
Estimated CL/F
5.7 L/hr
Metabolism
Primary Pathway
Nedosiran is metabolized by endo- and exonucleases to shorter oligonucleotides.
Excretion
Primary Pathway
Approximately 27% of the administered nedosiran dose is excreted unchanged into the urine within 24 hours of dosing.
a Nedosiran distributes primarily to the liver after subcutaneous administration.
Cmax = maximum plasma concentration; AUC0-last = area under the plasma concentration-time curve from time of administration (0) to the last measurable time point (last); Tmax = time to maximum concentration; Vz/F = apparent volume of distribution; CV = coefficient of variation; CL/F = apparent clearance.
Specific Populations
No clinically significant differences in the pharmacokinetics or pharmacodynamics of nedosiran were observed based on age (2 to 73 years old), sex, race/ethnicity, mild-to-moderate renal impairment (eGFR 30 to 89 mL/min/1.73 m2) [see Use in Specific Populations (8.7)] or mild hepatic impairment as assessed using the National Cancer Institute Organ Dysfunction Working Group criteria (total bilirubin ≤ ULN and AST > ULN; or total bilirubin >1 to 1.5 × ULN and any AST) [see Use in Specific Populations (8.6)].
Pediatrics:
At the recommended clinical dose, PK exposure of nedosiran is similar in adult and pediatric patients 2 years of age and older.
Drug Interaction Studies
Concomitant use of pyridoxine (vitamin B6) did not have a significant impact on the PK of nedosiran.
In vitro studies demonstrated that nedosiran was not an inhibitor or inducer of cytochrome P450 (CYP) enzymes and was neither a substrate nor an inhibitor of efflux and uptake transporters.
12.6 Immunogenicity
As with all oligonucleotides, including RIVFLOZA, there is a potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies in the studies described below with the incidence of antibodies in other studies or to other products may be misleading.
Across all clinical studies in the nedosiran development program, including patients with PH1 dosed with RIVFLOZA, RIVFLOZA did not induce or boost anti-drug antibodies (ADA). Among 79 patients tested with the ADA assay, none developed treatment-emergent ADA.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity
Long-term studies to assess carcinogenic risk of nedosiran have not been conducted.
Genotoxicity
Nedosiran was not genotoxic in the in vitro bacterial mutagenicity, in vitro micronucleus assays (human peripheral blood lymphocytes) and in vivo bone marrow micronucleus assay in mice.
Fertility
Weekly subcutaneous administration of nedosiran at doses of 500, 1000, or 2000 mg/kg or of a mouse-specific (pharmacologically active) analog at a dose of 10 mg/kg to male mice for 4 weeks prior to and throughout mating, and to female mice for 2 weeks prior to and throughout mating and to gestation day 7 did not affect male or female fertility or early embryonic development.
-
14 CLINICAL STUDIES
14.1 PHYOX2
PHYOX2 was a randomized, double-blind trial comparing RIVFLOZA and placebo in patients aged 6 years or older with PH1 or PH2 and an eGFR ≥ 30 mL/min/1.73 m2 (NCT03847909). Too few PH2 patients were enrolled to evaluate efficacy in the PH2 population. Therefore, RIVFLOZA is only indicated for patients with PH1 [see Indications and Usage (1)]. Unless otherwise noted, data are presented for the complete study population (PH1 and PH2).
Patients received monthly doses of RIVFLOZA (N=23) or placebo (N=12). The RIVFLOZA dose for patients at least 12 years of age weighing at least 50 kg was 160 mg, for patients at least 12 years of age weighing less than 50 kg was 128 mg, and for children 6 to 11 years of age was 3.3 mg/kg (to a maximum of 128 mg).
The median age was 20 years (range 9 - 46 years), 51% were female, 71% were White, 17% were Asian, 83% had PH1, and 17% had PH2. At baseline, mean 24-hour urinary oxalate excretion, normalized by 1.73 m2 BSA in patients less than 18 years of age, was 1547 µmol/24‑hour. Mean plasma oxalate was 8.2 µmol/L, 43% of patients had an eGFR ≥ 90 mL/min/1.73 m2, 34% had an eGFR 60 to < 90 mL/min/1.73 m2, 23% had an eGFR 30 to < 60 mL/min/1.73 m2, and 60% were taking pyridoxine.
The primary efficacy endpoint was the area under the curve, from Days 90 to 180, of the percent change from baseline in 24-hour urinary oxalate excretion (AUC24-hour Uox). The least-squares (LS) mean AUC24‑hour Uox was -3486 (95% CI: -5025, -1947) in the RIVFLOZA group compared to 1490 (95% CI: 781, 3761) in the placebo group, for a between group difference of 4976 (95% CI: 2803, 7149; p<0.0001).
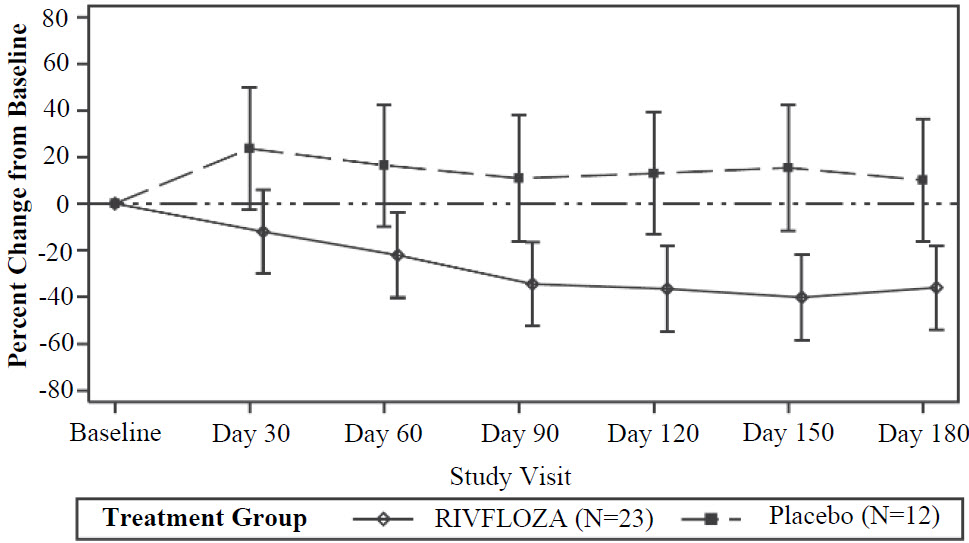
The LS mean percent change from baseline in 24-hour urinary oxalate excretion (corrected for BSA in patients < 18 years of age) averaged over Days 90, 120, 150 and 180, was -37% (95% CI: -53%, -21%) in the RIVFLOZA group and 12% (95% CI: ‑12%, 36%) in the placebo group, for a between group difference of 49% (95% CI: 26%, 72%) [Figure 1]. Among patients with PH1, the between group difference was 56% (95% CI: 33%, 80%).
Figure 1. Mean (95% CI) Percent Change from Baseline in 24-hour Urinary Oxalate in RIVFLOZA and Placebo-Treated Patients in PHYOX2
After 6 months of treatment in PHYOX2, patients could enroll in an ongoing single-arm extension study, PHYOX3 (NCT04042402), in which all patients were treated with RIVFLOZA. The reduction in urinary oxalate was maintained in the 13 patients with PH1 who received an additional 6 months of treatment in PHYOX3.
14.2 PHYOX8
PHYOX8 (NCT05001269) was a single-arm open-label multicenter study that included patients 2 years of age to less than 12 years of age with PH1 and an eGFR >30 mL/min/1.73 m2.
The median age of patients at first dose was 5 years (range 2 to 10 years), 33% were female, and 80% were White. A total of 15 patients with PH1 completed treatment; 8 patients were 2 to less than 6 years of age, 5 patients were 6 to less than 9 years of age and 2 patients were 9 to 11 years of age. The mean spot urinary oxalate:creatinine ratio at baseline was 0.36 mmol/mmol.
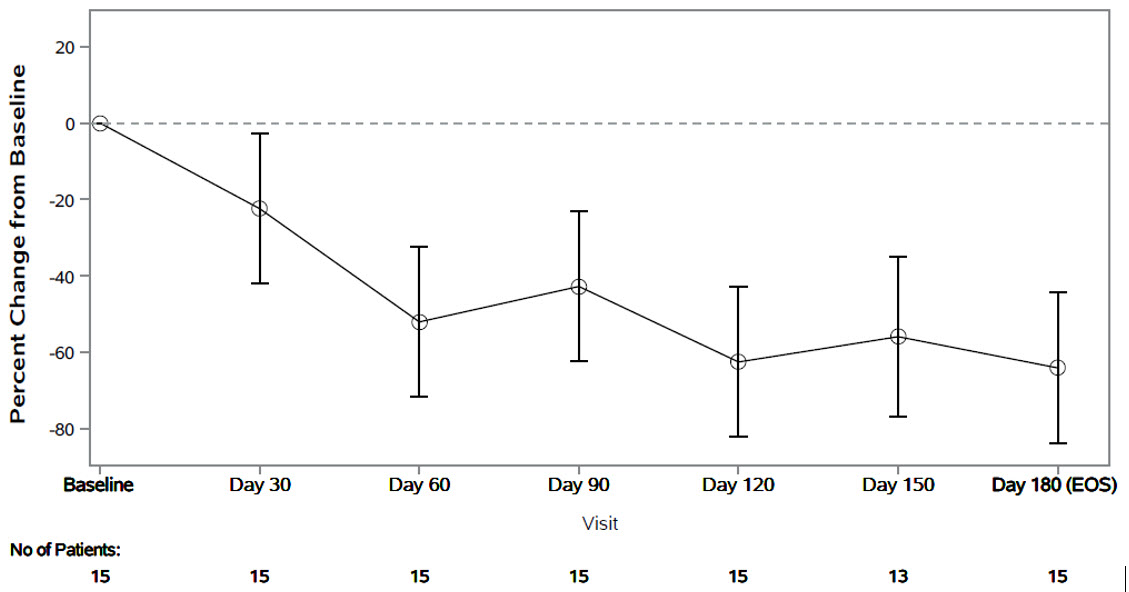
The primary endpoint was the percent change from baseline in spot urinary oxalate:creatinine ratio at Month 6. Patients treated with RIVFLOZA had a 64% (95% CI: 44, 84) reduction in spot urinary oxalate:creatinine ratio from baseline at Month 6 (Figure 2). The corresponding absolute reduction in spot urinary oxalate:creatinine ratio at Month 6 was 0.25 mmol/mmol (95% CI: 0.21, 0.29).
Figure 2. PHYOX8: Mean (95% CI) Percent Change in Spot Urinary Oxalate: Creatinine Ratio from Baseline by Month
After 6 months of treatment in PHYOX8, patients could enroll in an ongoing single arm extension study, PHYOX3. The reduction in urinary oxalate:creatinine ratio was maintained in the 8 patients who received an additional 6 months of treatment in PHYOX3.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
RIVFLOZA is a clear, sterile, preservative-free, colorless-to-yellow solution available in single-dose pre-filled syringes and single-dose vials in cartons containing one unit each.
- Table 3: RIVFLOZA Presentations
RIVFLOZA Presentation
Total Volume
Total amount available in presentation
Concentration
NDC number
Single-dose vial
0.5 mL
80 mg
160 mg/mL
NDC: 0169-5308-01
Single-dose
Pre-filled Syringe
0.8 mL
128 mg
160 mg/mL
NDC: 0169-5307-08
Single-dose
Pre-filled Syringe
1 mL
160 mg
160 mg/mL
NDC: 0169-5306-10
16.2 Storage and Handling
Store refrigerated at 2°C to 8°C (36°F to 46°F). RIVFLOZA can be stored, if needed, at 15°C to 30°C (59°F to 86°F) for a maximum of 28 days (4 weeks). Do not freeze. Store in original carton, away from direct heat and light.
- Table 4: Storage Conditions for RIVFLOZA
Refrigerated
2°C to 8°C (36°F to 46°F)
Room Temperature at 15°C to 30°C
(59°F to 86°F)
RIVFLOZA
Until expiration date
Maximum 28 days (4 weeks)
-
17 PATIENT COUNSELING INFORMATION
- Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
- Instruct patients/caregivers on the appropriate dose of RIVFLOZA to use, the timing of the dose, how and where to inject subcutaneously, and what to do if a dose is missed.
For more information contact:
Dicerna Pharmaceuticals, Inc.
A Novo Nordisk companyNovo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536
1-844-906-5099Manufactured by
Pyramid Laboratories
3598 Cadillac Ave
Costa Mesa, CA 92626 -
Patient Package Insert
PATIENT INFORMATION
RIVFLOZA® (Riv-flo-za)
(nedosiran)
injection, for subcutaneous use
What is RIVFLOZA?
RIVFLOZA is a prescription medicine used to lower urinary oxalate levels in children 2 years of age and older and adults with primary hyperoxaluria type 1 (PH1) and relatively preserved kidney function.
It is not known if RIVFLOZA is safe and effective in children younger than 2 years of age.
Before using RIVFLOZA, tell your healthcare provider about all of your medical conditions, including if you:
- are pregnant or plan to become pregnant. It is not known if RIVFLOZA will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if RIVFLOZA passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby during treatment with RIVFLOZA.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I use RIVFLOZA?
- Read the detailed Instructions for Use that comes with RIVFLOZA about the right way to prepare and inject RIVFLOZA.
- Use RIVFLOZA exactly as your healthcare provider tells you to.
- Inject RIVFLOZA under your skin (subcutaneous injection).
- Use RIVFLOZA 1 time each month.
- Your healthcare provider will prescribe the dose of RIVFLOZA that is right for you or your child based on your or your child’s body weight.
- RIVFLOZA comes as a single-dose Pre-filled Syringe and as a single-dose vial.
- Your healthcare provider will show you or your caregiver how to prepare and inject RIVFLOZA. Do not try to inject RIVFLOZA until you or your caregiver have been shown the right way by your healthcare provider.
- In children 2 years of age to less than 12 years of age weighing 86 pounds (39 kilograms) or more, it is recommended that RIVFLOZA Pre-filled Syringe be given by a healthcare provider or caregiver.
- If you miss a dose of RIVFLOZA, inject the dose as soon as possible. If you miss a dose of RIVFLOZA by more than 7 days, inject the dose as soon as possible and resume monthly dosing from the most recently injected dose. If you have any questions about a missed dose, call your healthcare provider or pharmacist.
What are the possible side effects of RIVFLOZA?
The most common side effects of RIVFLOZA include injection site reactions, such as reddening, pain, bruising, rash, or dimple at the site of injection.
These are not all the possible side effects of RIVFLOZA.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
You may also report side effects to Novo Nordisk at 1-844-906-5099.
How should I store RIVFLOZA?
- Store RIVFLOZA in the refrigerator between 36°F to 46°F (2°C to 8°C).
- If needed, RIVFLOZA can be stored between 59°F to 86°F (15°C to 30°C) for up to 28 days (4 weeks). Record the date RIVFLOZA was removed from the refrigerator on the carton and throw away (dispose of) if not used within 28 days.
- Do not freeze RIVFLOZA.
- Store RIVFLOZA in the original carton.
- Keep RIVFLOZA away from direct heat and light.
Keep RIVFLOZA and all medicines out of the reach of children.
General information about the safe and effective use of RIVFLOZA.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use RIVFLOZA for a condition for which it was not prescribed. Do not give RIVFLOZA to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about RIVFLOZA that is written for health professionals.
What are the ingredients in RIVFLOZA?
Active ingredient: nedosiran
Inactive ingredients: water for injection and sodium hydroxide and/or hydrochloric acid.
For more information contact: Dicerna Pharmaceuticals, Inc., A Novo Nordisk company
Novo Nordisk Inc., 800 Scudders Mill Road, Plainsboro, NJ 08536
1-844-906-5099
Manufactured by: Pyramid Laboratories, 3598 Cadillac Ave, Costa Mesa, CA 92626
For more information, go to https://www.rivfloza.com/ or call 1-844-906-5099.
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 03/2025
-
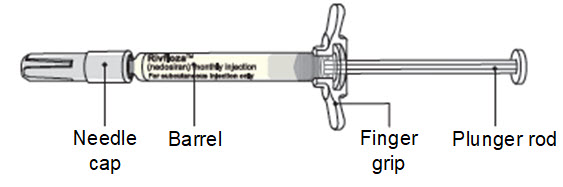
Instructions for Use – Pre-filled Syringe
-
Instructions for Use - Vial
INSTRUCTIONS FOR USE
RIVFLOZA® (Riv-flo-za)
(nedosiran)
injection, for subcutaneous use
Single-dose vial
This Instructions for Use contains information on how to inject RIVFLOZA using the single-dose vial in:
- children 2 years of age to less than 12 years of age weighing less than 86 pounds (39 kilograms).
- adults and children using the single-dose vial as an alternative to the single-dose Pre-filled syringe.
Read the Instructions for Use before using RIVFLOZA vial and each time you get a refill. There may be new information. Ask your or your child’s healthcare provider if you have any questions.
Important information you need to know before injecting RIVFLOZA.
- Your or your child’s healthcare provider will show you how to prepare and inject RIVFLOZA. Do not try to inject RIVFLOZA until you have been shown the right way by your or your child’s healthcare provider.
- Use RIVFLOZA vials exactly as your or your child’s healthcare provider tells you to.
- Your or your child’s healthcare provider will tell you how much RIVFLOZA to inject and when to inject it.
- Do not use the RIVFLOZA vial if the carton is damaged or if the tamper-proof seal is not intact.
- Do not use the RIVFLOZA vial if the expiration date on the carton has passed.
- Uncap the RIVFLOZA vial only when ready to give an injection. Under the vial cap, you will see a grey rubber stopper. Do not remove it. It is supposed to be there.
- RIVFLOZA vials are for one-time use (single-dose) only. Do not reuse the RIVFLOZA vial. Throw away (discard of) any unused RIVFLOZA.
- RIVFLOZA is for injection under the skin (subcutaneous injection) only. Do not inject RIVFLOZA into a vein.
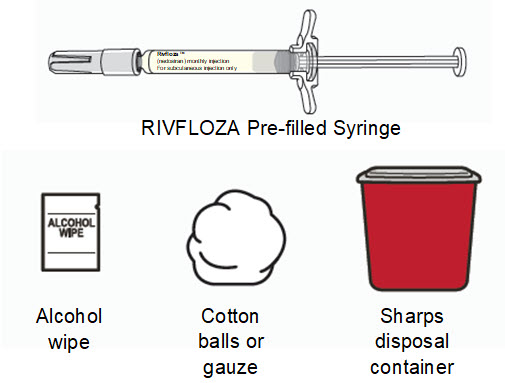
Supplies needed to give the injection
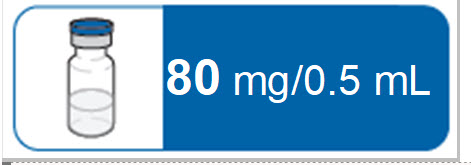
If the amount of RIVFLOZA needed for your or your child’s prescribed dose is 0.5 mL or less, you will need the following supplies:
- 1 RIVFLOZA vial
The following supplies are not included in the carton:
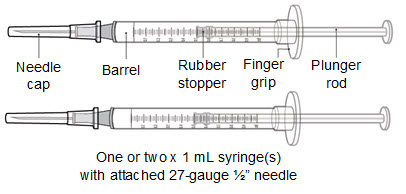
- One 1 mL syringe with attached 27-gauge 1/2” needle
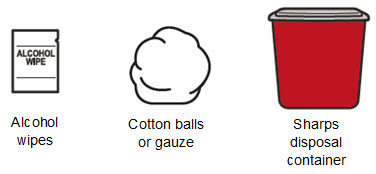
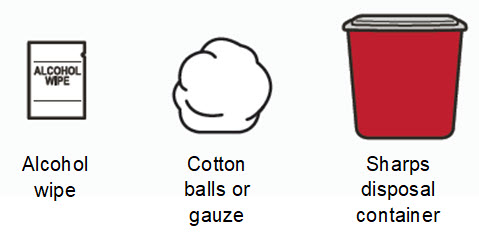
- Alcohol wipes
- Cotton balls or gauze
- Puncture resistant sharps disposal container. See Step 16 “Throw away (dispose of) the used syringe(s)” at the end of this Instructions for Use.
If the amount of RIVFLOZA needed for your or your child’s prescribed dose is 0.6 mL or more, you will need the following supplies:
- 2 RIVFLOZA vials
The following supplies are not included in the carton:
- Two 1 mL syringes with attached 27-gauge 1/2” needle
- Alcohol wipes
- Cotton balls or gauze
- Puncture resistant sharps disposal container. See Step 16 “Throw away (dispose of) the used syringe(s)” at the end of this Instructions for Use.
How should I store RIVFLOZA vials?
- Store RIVFLOZA vials in the refrigerator between 36°F to 46°F (2°C to 8°C).
- If needed, RIVFLOZA vials may be stored between 59°F to 86°F (15°C to 30°C) for no longer than 28 days (4 weeks). Record the date RIVFLOZA was removed from the refrigerator on the carton and throw away (dispose of) if not used within 28 days.
- Store RIVFLOZA in the original carton.
- Keep RIVFLOZA vials away from direct heat and light.
- Do not freeze.
Keep RIVFLOZA vials and all medicines out of the reach of children.
A. Preparing for the injection
Step 1. Gather the supplies and place the supplies on a clean, flat surface in a well-lit area.
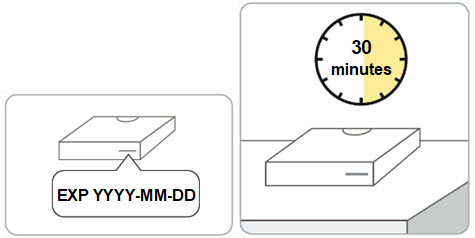
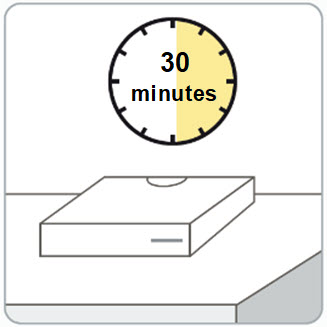
Step 2. Remove the RIVFLOZA vial carton(s) from the refrigerator.
- Check the expiration date on the carton. Do not use if the expiration date on the carton has passed.
- Wait 30 minutes before injecting to allow the medicine in the vial to warm to room temperature.
Caution:
- Keep the RIVFLOZA vial in the carton and out of direct heat and sunlight.
- Do not warm the vial using any heat sources such as hot water or a microwave.
Step 3. Wash your hands with soap and water.
Step 4. Open the carton(s) and remove the RIVFLOZA vial(s).
- Check the vial label to make sure you have the correct medicine for the prescription.
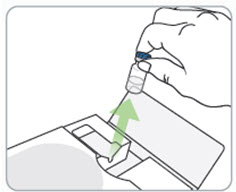
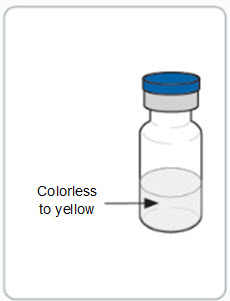
Step 5. Inspect the RIVFLOZA vial(s).
-
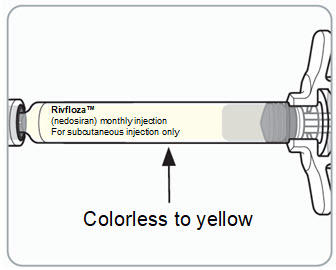
Look at the medicine in the vial. It should be colorless to yellow and free of particles.
- o Do not use the vial if the medicine looks cloudy, discolored, or contains particles.
-
The vial should not look damaged.
- o Do not use the vial if the vial looks damaged.
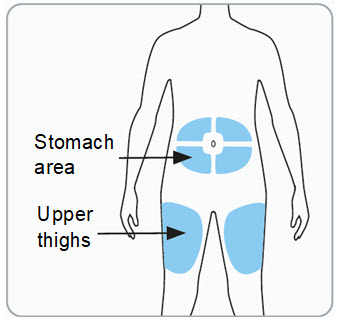
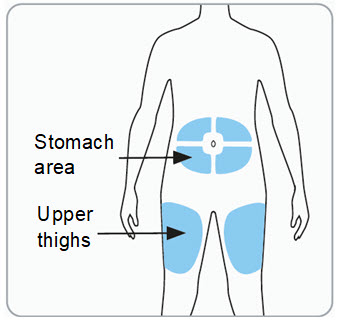
Step 6. Choose the injection site(s).
-
You may inject into the skin of:
- o the stomach area (abdomen) at least 2 inches from the belly button, or
- o the upper thigh.
- If the dose is more than 0.5 mL (2 injections), inject the contents of each syringe in a different location. If both injections are in the abdomen, they should be in different areas of the abdomen.
Caution:
- Do not inject into scarred or bruised skin.
- Do not inject the contents of 2 syringes into the same location.
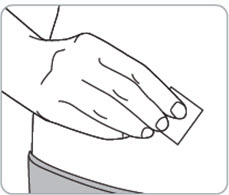
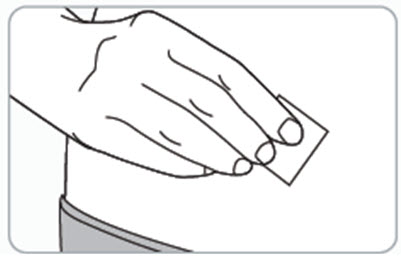
Step 7. Clean the injection site(s).
- Clean the injection site(s) with an alcohol wipe and let it air dry.
- Do not touch, wipe with other material, fan, or blow on the cleaned injection site(s).
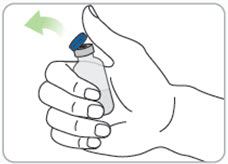
Step 8. Prepare the vial(s).
- Remove the cap from the vial(s) you will need.
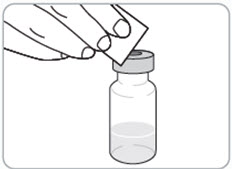
- Clean the top of the grey rubber stopper with a new alcohol wipe.
Caution:
- Do not remove the grey rubber stopper from the vial. It is supposed to be there.
B. Giving the injection
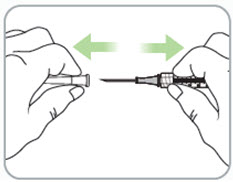
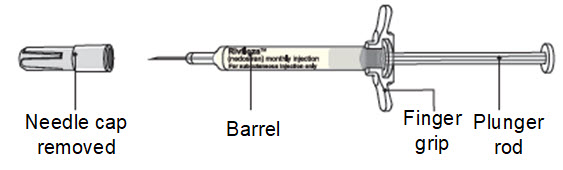
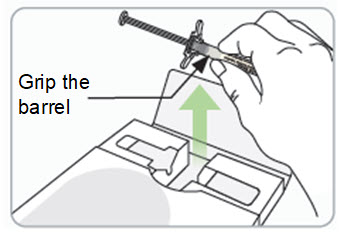
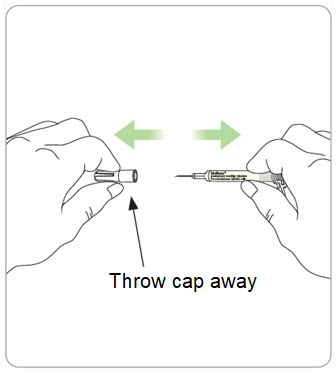
Step 9. Remove the syringe with attached needle from the packaging and remove the needle cap. Throw the needle cap away in the sharps disposal container.
- Remove the needle cap by pulling it straight off and away from your body.
Caution:
- Take care when handling the uncapped needle.
- Do not touch the uncapped needle.
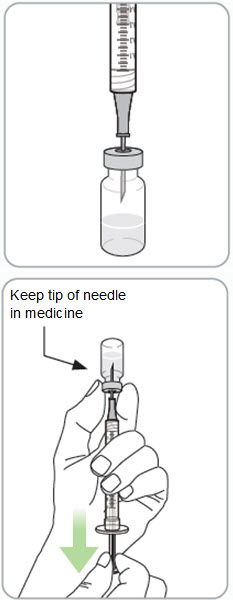
Step 10. Withdraw RIVFLOZA into the syringe.
If the dose amount is 0.5 mL or less:
- Insert the needle into the grey rubber stopper on top of the vial.
- Turn the vial and syringe upside down.
- Keep the tip of the needle in the medicine.
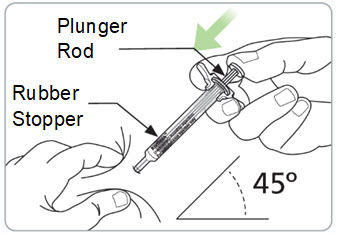
- Hold the syringe and vial in 1 hand. With your other hand, slowly pull back on the plunger rod to withdraw the prescribed dose into the syringe.
If the dose amount is 0.6 mL or more:
- You will need to withdraw the dose of RIVFLOZA from 2 vials using 2 separate syringes.
- Follow “If the dose amount is 0.5 mL or less” instructions to withdraw the amount of medicine needed from each vial as instructed by your or your child’s healthcare provider. Then follow Steps 11 to 16 for each syringe to inject RIVFLOZA.
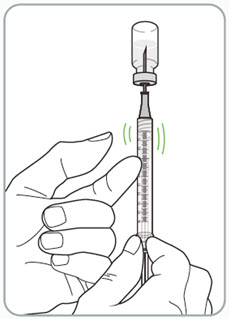
Step 11. Remove any large air bubbles from the syringe.
- If you see large air bubbles in the syringe, tap the side of the syringe to move any air bubbles to the top of the syringe.
- Push the plunger rod up to push the air bubbles back into the vial.
- If the syringe does not contain the correct dose after the large air bubbles are removed, you will need to pull back on the plunger rod again to fill the syringe with the prescribed dose.
- Look at the syringe to make sure you have the correct amount for the dose.
Caution:
- Be sure the syringe is free of large air bubbles before you inject.
Step 12. Turn the vial and syringe back upright and remove the needle from the vial.
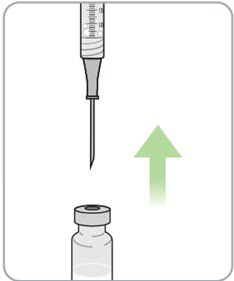
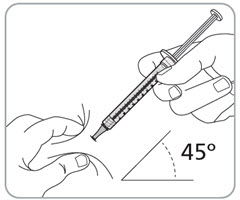
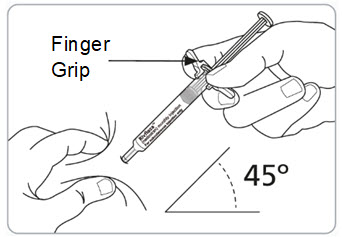
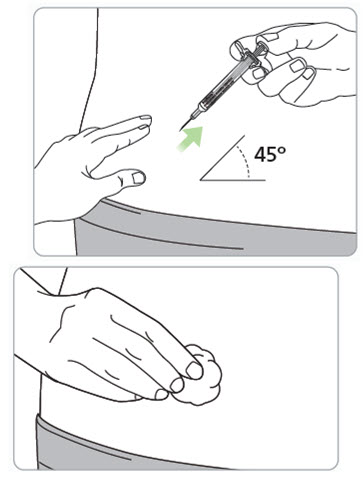
Step 13. Pinch the skin and fully insert the needle.
- Pinch the skin around the injection site with 1 hand.
- With your other hand, fully insert the needle into the skin at a 45-degree angle.
- If you are giving 2 injections, inject each syringe in a different location. If both injections are in the abdomen, they should be in different areas of the abdomen.
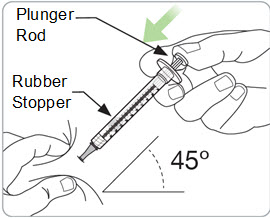
Step 14. Slowly inject all the medicine.
- Gently push the plunger rod all the way down until the syringe is empty.
- You will see the rubber stopper inside the syringe move to the bottom of the barrel as the medicine is injected.
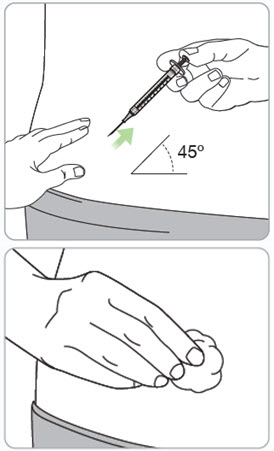
Step 15. Remove the needle from the injection site.
Caution:
- Do not recap the needle.
- Do not save or keep used syringes.
Throw away (dispose of) the used vial(s) in household trash.
If there is bleeding, lightly press a cotton ball or gauze over the injection site.
C. After the injection
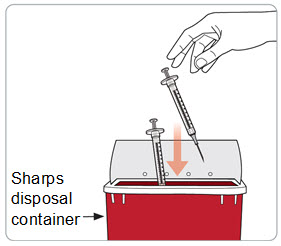
Step 16. Throw away (dispose of) the used syringe(s).
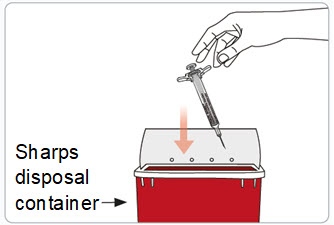
- Put the used syringe(s) with the needle still attached in an FDA-cleared sharps container right away after use.
- Do not throw away (dispose of) needles and syringes in your household trash.
- If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- o made of a heavy-duty plastic,
- o can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- o upright and stable during use,
- o leak-resistant, and
- o properly labeled to warn of hazardous waste inside the container.
- When the sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes.
- For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal
- Do not dispose of the used sharps disposal container in your household trash unless your community guidelines permit this.
- Do not recycle the used sharps disposal container.
Frequently asked questions
What if a dose is missed?
- If a dose of RIVFLOZA is missed, inject the dose as soon as possible.
- If a dose of RIVFLOZA is missed by more than 7 days, inject the RIVFLOZA dose as soon as possible and resume monthly dosing from the most recently injected dose.
If you have any questions about a missed dose, call your healthcare provider or pharmacist.
What if I damage or break the RIVFLOZA vial?
- Do not use a broken or damaged vial. Call the pharmacy for a replacement.
Do I inject the full volume of the vial?
- The amount of RIVFLOZA you will inject depends on the prescribed dose. You may need more than one vial, one vial, or less than one vial for the prescribed dose.
What if I need 2 vials for injection?
- Use 2 separate syringes and withdraw the amount from each vial as directed by your or your child’s healthcare provider. Give 2 separate injections in a different location. If both injections are in the abdomen, they should be in different areas of the abdomen.
“Pull Out” panel (on front of IFU)

INSTRUCTIONS FOR USE
RIVFLOZA® (Riv-flo-za)
(nedosiran)
injection, for subcutaneous use
Single-dose vial
Address Panel
For more information go to https://www.rivfloza.com/ or call 1-844-906-5099.
Dicerna Pharmaceuticals, Inc.
A Novo Nordisk company
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536 USA
1-888-906-5099
Manufactured by:
Pyramid Laboratories
3598 Cadillac Avenue
Costa Mesa, CA 92626 USA
Version: 3
© 2025 Novo Nordisk
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: 03/2025
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – Pre-filled Syringe 128 mg/0.8 mL
NDC: 0169-5307-08
List 530708
rivfloza™
(nedosiran) injection
128 mg/0.8 mL
For subcutaneous injection only
1 x 0.8 mL Sterile Single-dose Pre-filled Syringe
Do not use the Pre-filled Syringe if the carton is damaged or if the tamper proof seal is not intact.
Rx Only
Dicerna™
a Novo Nordisk company
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – Pre-filled Syringe 160 mg/mL
NDC: 0169-5306-10
List 530610
rivfloza™
(nedosiran) injection
160 mg/mL
For subcutaneous injection only
1 x 1 mL Sterile Single-dose Pre-filled Syringe
Do not use the Pre-filled Syringe if the carton is damaged or if the tamper proof seal is not intact.
Rx Only
Dicerna™
a Novo Nordisk company
-
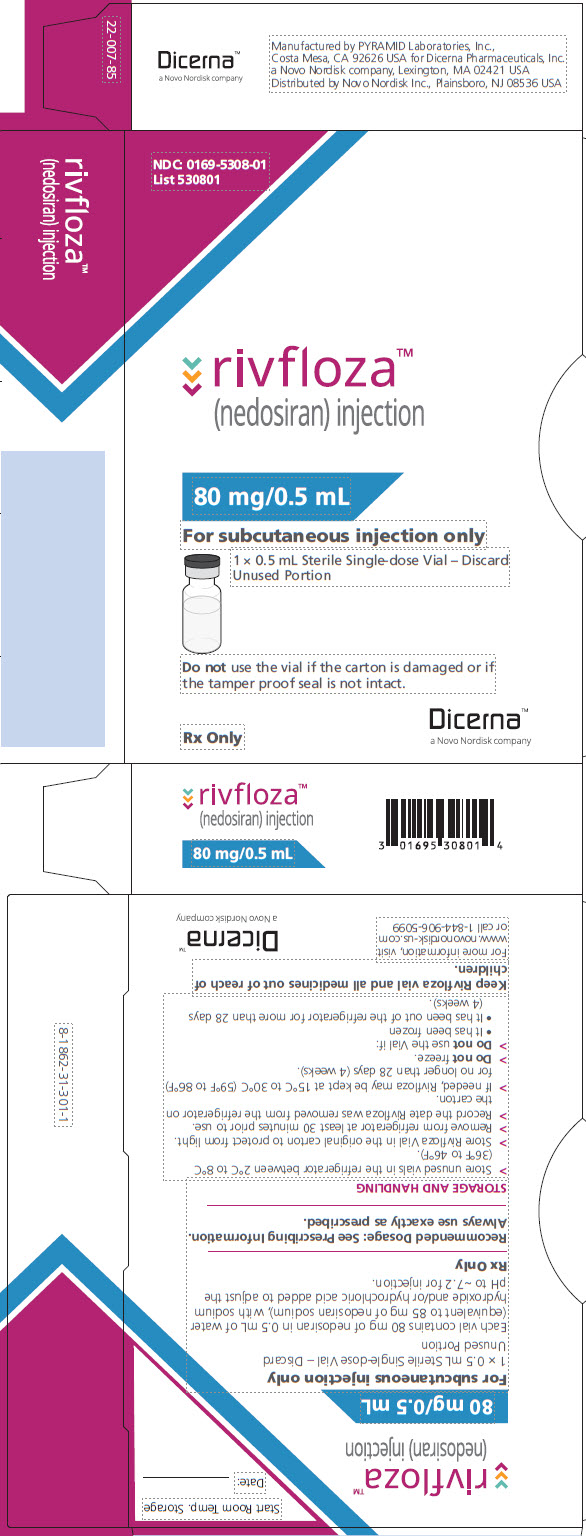
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – Vial 80 mg/0.5 mL
NDC: 0169-5308-01
List 530801
rivfloza™
(nedosiran) injection
80 mg/0.5 mL
For subcutaneous injection only
1 x 0.5 mL Sterile Single-dose Vial – Discard Unused Portion
Do not use the vial if the carton is damaged or if the tamper proof seal is not intact.
Rx Only
Dicerna™
a Novo Nordisk company
-
INGREDIENTS AND APPEARANCE
RIVFLOZA
nedosiran injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0169-5306 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NEDOSIRAN SODIUM (UNII: EGR9KYM536) (NEDOSIRAN - UNII:13U9R5J3WL) NEDOSIRAN 160 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Other Ingredients Ingredient Kind Ingredient Name Quantity May contain SODIUM HYDROXIDE (UNII: 55X04QC32I) May contain HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0169-5306-10 1 in 1 CARTON 02/19/2024 1 1 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA215842 02/19/2024 RIVFLOZA
nedosiran injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0169-5307 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NEDOSIRAN SODIUM (UNII: EGR9KYM536) (NEDOSIRAN - UNII:13U9R5J3WL) NEDOSIRAN 128 mg in 0.8 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Other Ingredients Ingredient Kind Ingredient Name Quantity May contain SODIUM HYDROXIDE (UNII: 55X04QC32I) May contain HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0169-5307-08 1 in 1 CARTON 02/19/2024 1 0.8 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA215842 02/19/2024 RIVFLOZA
nedosiran injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0169-5308 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NEDOSIRAN SODIUM (UNII: EGR9KYM536) (NEDOSIRAN - UNII:13U9R5J3WL) NEDOSIRAN 80 mg in 0.5 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Other Ingredients Ingredient Kind Ingredient Name Quantity May contain SODIUM HYDROXIDE (UNII: 55X04QC32I) May contain HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0169-5308-01 1 in 1 CARTON 02/19/2024 1 0.5 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA215842 02/19/2024 Labeler - Novo Nordisk (622920320)
Trademark Results [RIVFLOZA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 RIVFLOZA 90191150 not registered Live/Pending |
Dicerna Pharmaceuticals, Inc. 2020-09-18 |
 RIVFLOZA 79365572 not registered Live/Pending |
NOVO NORDISK HEALTH CARE AG 2023-02-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.


 Read Entire Instructions Before Use
Read Entire Instructions Before Use Follow Instructions Carefully
Follow Instructions Carefully Contact Novo Nordisk for any Questions
Contact Novo Nordisk for any Questions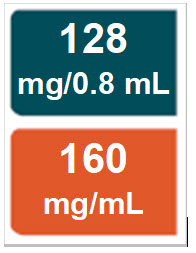


 Read Entire Instructions Before Use
Read Entire Instructions Before Use Follow Instructions Carefully
Follow Instructions Carefully Contact Novo Nordisk for any Questions
Contact Novo Nordisk for any Questions