TOUCHLAND POWER MIST HYDRATING HAND SANITIZER LIMITED EDITION SEASONAL FIVE SET- alcohol kit
Touchland Power Mist Hydrating Hand Sanitizer Limited Edition Seasonal Five Set by
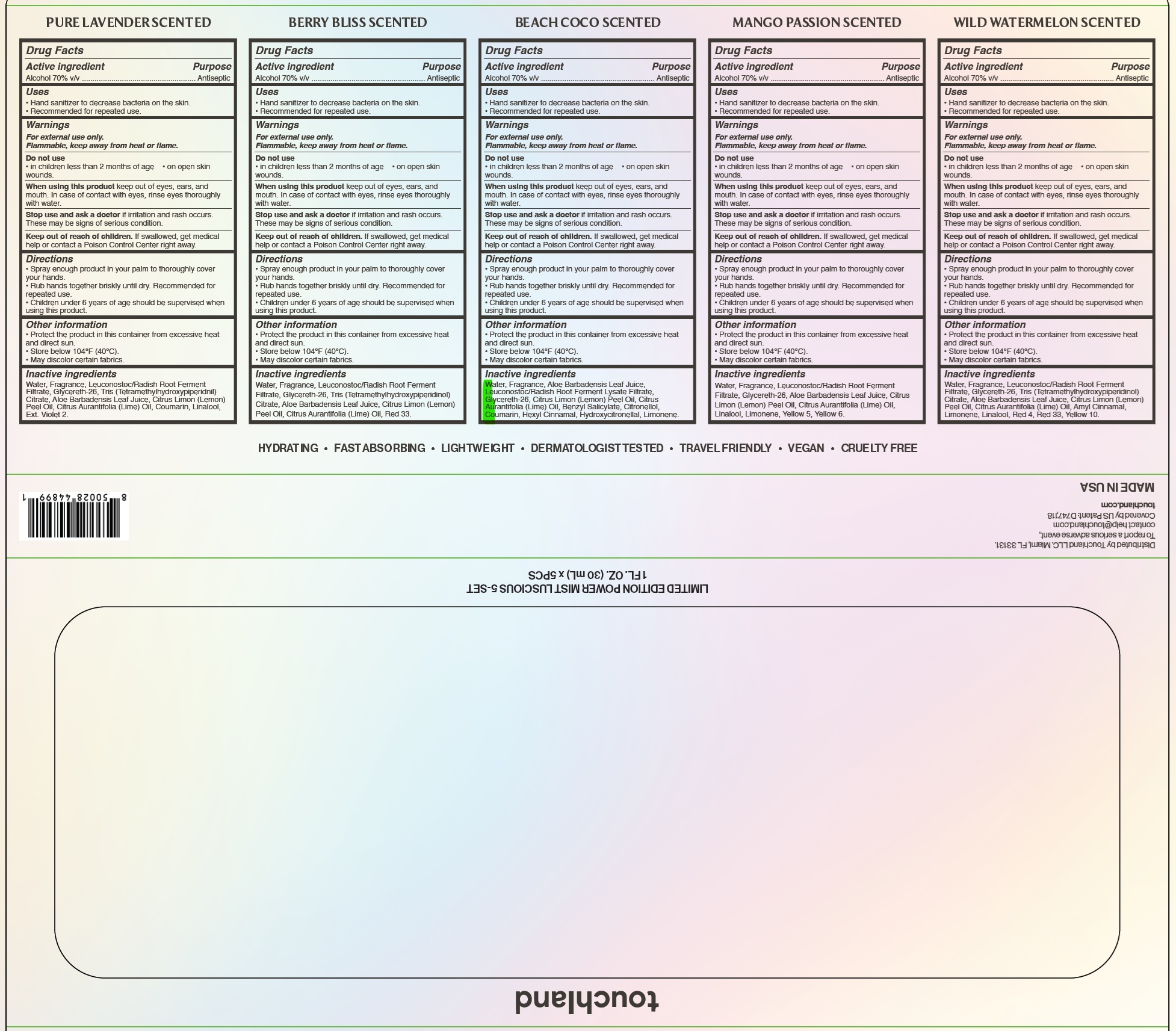
Drug Labeling and Warnings
Touchland Power Mist Hydrating Hand Sanitizer Limited Edition Seasonal Five Set by is a Otc medication manufactured, distributed, or labeled by TOUCHLAND LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Drug Facts
- Active ingredient
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Drug Facts
- Active ingredient
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Drug Facts
- Active ingredient
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Drug Facts
- Active ingredient
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Drug Facts
- Active ingredient
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Package Labeling:
- Pure Lavander
- Berry Bliss
- Beach Coco
- Mango
- Watermelon
-
INGREDIENTS AND APPEARANCE
TOUCHLAND POWER MIST HYDRATING HAND SANITIZER LIMITED EDITION SEASONAL FIVE SET
alcohol kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 72033-149 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72033-149-00 1 in 1 KIT 08/01/2025 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 30 mL Part 2 1 BOTTLE 30 mL Part 3 1 BOTTLE, SPRAY 30 mL Part 4 1 BOTTLE 30 mL Part 5 1 BOTTLE 30 mL Part 1 of 5 TOUCHLAND POWER MIST PURE LAVANDER SCENTED HYDRATING HAND SANITIZER
alcohol sprayProduct Information Item Code (Source) NDC: 72033-134 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) LEUCONOSTOC/RADISH ROOT FERMENT FILTRATE (UNII: D2QHA03458) GLYCERETH-26 (UNII: NNE56F2N14) TRIS(TETRAMETHYLHYDROXYPIPERIDINOL) CITRATE (UNII: 7NW772I64Y) ALOE VERA LEAF JUICE (UNII: RUE8E6T4NB) LEMON OIL, COLD PRESSED (UNII: I9GRO824LL) LIME OIL, COLD PRESSED (UNII: UZH29XGA8G) COUMARIN (UNII: A4VZ22K1WT) LINALOOL, (+/-)- (UNII: D81QY6I88E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72033-134-00 1 in 1 CARTON 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 08/01/2025 Part 2 of 5 TOUCHLAND POWER MIST BERRY BLISS SCENTED HYDRATING HAND SANITIZER
alcohol sprayProduct Information Item Code (Source) NDC: 72033-135 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) LEUCONOSTOC/RADISH ROOT FERMENT FILTRATE (UNII: D2QHA03458) GLYCERETH-26 (UNII: NNE56F2N14) TRIS(TETRAMETHYLHYDROXYPIPERIDINOL) CITRATE (UNII: 7NW772I64Y) ALOE VERA LEAF JUICE (UNII: RUE8E6T4NB) LEMON OIL, COLD PRESSED (UNII: I9GRO824LL) LIME OIL, COLD PRESSED (UNII: UZH29XGA8G) D&C RED NO. 33 (UNII: 9DBA0SBB0L) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72033-135-00 1 in 1 CARTON 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 08/01/2025 Part 3 of 5 TOUCHLAND POWER MIST HAND SANITIZER BEACH COCO
alcohol sprayProduct Information Item Code (Source) NDC: 72033-110 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALOE VERA LEAF JUICE (UNII: RUE8E6T4NB) GLYCERETH-26 (UNII: NNE56F2N14) LEMON OIL, COLD PRESSED (UNII: I9GRO824LL) LIME OIL, COLD PRESSED (UNII: UZH29XGA8G) BENZYL SALICYLATE (UNII: WAO5MNK9TU) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) COUMARIN (UNII: A4VZ22K1WT) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) HYDROXYCITRONELLAL (UNII: 8SQ0VA4YUR) LIMONENE, (+)- (UNII: GFD7C86Q1W) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72033-110-01 30 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 08/01/2025 Part 4 of 5 TOUCHLAND POWER MIST MANGO PASSION SCENTED HYDRATING HAND SANITIZER
alcohol sprayProduct Information Item Code (Source) NDC: 72033-137 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) LEUCONOSTOC/RADISH ROOT FERMENT FILTRATE (UNII: D2QHA03458) GLYCERETH-26 (UNII: NNE56F2N14) ALOE VERA LEAF JUICE (UNII: RUE8E6T4NB) LEMON OIL, COLD PRESSED (UNII: I9GRO824LL) LIME OIL, COLD PRESSED (UNII: UZH29XGA8G) LINALOOL, (+/-)- (UNII: D81QY6I88E) LIMONENE, (+)- (UNII: GFD7C86Q1W) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72033-137-00 1 in 1 CARTON 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 08/01/2025 Part 5 of 5 TOUCHLAND POWER MIST WILD WATERMELON SCENTED HYDRATING HAND SANITIZER
alcohol sprayProduct Information Item Code (Source) NDC: 72033-138 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) LEUCONOSTOC/RADISH ROOT FERMENT FILTRATE (UNII: D2QHA03458) GLYCERETH-26 (UNII: NNE56F2N14) TRIS(TETRAMETHYLHYDROXYPIPERIDINOL) CITRATE (UNII: 7NW772I64Y) ALOE VERA LEAF JUICE (UNII: RUE8E6T4NB) LEMON OIL, COLD PRESSED (UNII: I9GRO824LL) LIME OIL, COLD PRESSED (UNII: UZH29XGA8G) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) FD&C RED NO. 4 (UNII: X3W0AM1JLX) D&C RED NO. 33 (UNII: 9DBA0SBB0L) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72033-138-00 1 in 1 CARTON 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 08/01/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 08/01/2025 Labeler - TOUCHLAND LLC (036656461)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.