Cycle by La Mend, Inc / Active Intelligence LLC
Cycle by
Drug Labeling and Warnings
Cycle by is a Otc medication manufactured, distributed, or labeled by La Mend, Inc, Active Intelligence LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
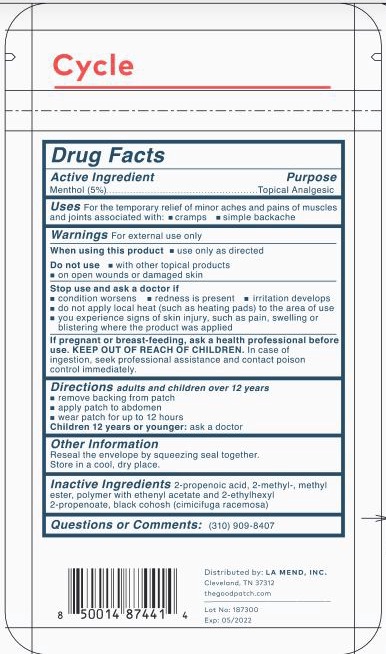
CYCLE- menthol patch
La Mend, Inc
----------
UsesFor the temporary relief of minor aches and pains of muscles and joints associated with
- cramps
- simple backache
WarningsFor external use only
Directionsadults and children over 12 years
- remove backing from patch
- apply patch to abdomen
- wear patch for up to 12 hours
Children 12 years or younger: ask a doctor
Inactive Ingredients2-propenoic acid, 2-methyl-, methyl ester, polymer with ethenyl acetate and 2-ethylhexyl 2-propenoate, black cohosh (cimicifuga racemosa)
| CYCLE
menthol patch |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - La Mend, Inc (117940830) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Active Intelligence LLC | 080416593 | manufacture(72587-110) | |
Revised: 12/2023
<
Document Id: 0cfc399e-7734-2a28-e063-6294a90a2f16
Set id: ad3dfa40-e33a-5087-e053-2995a90abbbc
Version: 4
Effective Time: 20231220
Trademark Results [Cycle]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CYCLE 98806528 not registered Live/Pending |
FRIZ Biochem GmbH 2024-10-17 |
 CYCLE 98720094 not registered Live/Pending |
Renew Residential, Inc. 2024-08-27 |
 CYCLE 98676715 not registered Live/Pending |
Cycle Pharmaceuticals Ltd 2024-07-31 |
 CYCLE 88610511 not registered Live/Pending |
Cycle Technology, Inc. 2019-09-10 |
 CYCLE 88610508 not registered Live/Pending |
Cycle Technology, Inc. 2019-09-10 |
 CYCLE 88258272 not registered Dead/Abandoned |
Samson, Jessica 2019-01-11 |
 CYCLE 88239136 not registered Live/Pending |
Cycle Water Ltd. 2018-12-21 |
 CYCLE 88157136 5930142 Live/Registered |
Opal Talent Management LLC 2018-10-16 |
 CYCLE 87827612 not registered Dead/Abandoned |
Future Audio Workshop 2018-03-09 |
 CYCLE 87686478 not registered Live/Pending |
Cycle Collective, LLC 2017-11-15 |
 CYCLE 87686472 not registered Live/Pending |
Cycle Collective, LLC 2017-11-15 |
 CYCLE 87686468 not registered Live/Pending |
Cycle Collective, LLC 2017-11-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.